Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes
- PMID: 29634344
- PMCID: PMC6277084
- DOI: 10.1089/ars.2017.7269
Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes
Abstract
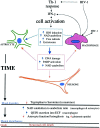
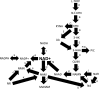
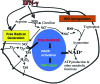
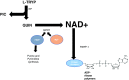
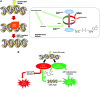
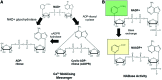
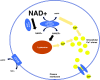
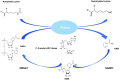
Significance: Nicotinamide adenine dinucleotide (NAD+) is an essential pyridine nucleotide that serves as an essential cofactor and substrate for a number of critical cellular processes involved in oxidative phosphorylation and ATP production, DNA repair, epigenetically modulated gene expression, intracellular calcium signaling, and immunological functions. NAD+ depletion may occur in response to either excessive DNA damage due to free radical or ultraviolet attack, resulting in significant poly(ADP-ribose) polymerase (PARP) activation and a high turnover and subsequent depletion of NAD+, and/or chronic immune activation and inflammatory cytokine production resulting in accelerated CD38 activity and decline in NAD+ levels. Recent studies have shown that enhancing NAD+ levels can profoundly reduce oxidative cell damage in catabolic tissue, including the brain. Therefore, promotion of intracellular NAD+ anabolism represents a promising therapeutic strategy for age-associated degenerative diseases in general, and is essential to the effective realization of multiple benefits of healthy sirtuin activity. The kynurenine pathway represents the de novo NAD+ synthesis pathway in mammalian cells. NAD+ can also be produced by the NAD+ salvage pathway. Recent Advances: In this review, we describe and discuss recent insights regarding the efficacy and benefits of the NAD+ precursors, nicotinamide (NAM), nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN), in attenuating NAD+ decline in degenerative disease states and physiological aging. Critical Issues: Results obtained in recent years have shown that NAD+ precursors can play important protective roles in several diseases. However, in some cases, these precursors may vary in their ability to enhance NAD+ synthesis via their location in the NAD+ anabolic pathway. Increased synthesis of NAD+ promotes protective cell responses, further demonstrating that NAD+ is a regulatory molecule associated with several biochemical pathways. Future Directions: In the next few years, the refinement of personalized therapy for the use of NAD+ precursors and improved detection methodologies allowing the administration of specific NAD+ precursors in the context of patients' NAD+ levels will lead to a better understanding of the therapeutic role of NAD+ precursors in human diseases.
Keywords: DNA damage; NAD; nicotinamide; oxidative stress; sirtuins.
Figures


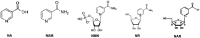
References
-
- Abeti R. and Duchen MR. Activation of PARP by oxidative stress induced by beta-amyloid: implications for Alzheimer's disease. Neurochem Res 37: 2589–2596, 2012 - PubMed
-
- Agote M, Viaggi M, Kreimann E, Krawiec L, Dagrosa , Juvenal GJ, Pisarev Influence of nicotinamide on the radiosensitivity of normal and goitrous thyroid in the rat. Thyroid 11: 1003–1007, 2001 - PubMed
-
- Agote Robertson M, Finochietto P, Gamba CA, Dagrosa MA, Viaggi ME, Franco MC, Poderoso JJ, Juvenal GJ, and Pisarev MA. Nicotinamide increases thyroid radiosensitivity by stimulating nitric oxide synthase expression and the generation of organic peroxides. Horm Metab Res 38: 12–15, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

