A Triazole Disulfide Compound Increases the Affinity of Hemoglobin for Oxygen and Reduces the Sickling of Human Sickle Cells
- PMID: 29634905
- PMCID: PMC5942180
- DOI: 10.1021/acs.molpharmaceut.8b00108
A Triazole Disulfide Compound Increases the Affinity of Hemoglobin for Oxygen and Reduces the Sickling of Human Sickle Cells
Abstract
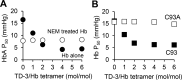
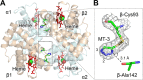
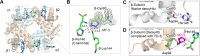
Sickle cell disease is an inherited disorder of hemoglobin (Hb). During a sickle cell crisis, deoxygenated sickle hemoglobin (deoxyHbS) polymerizes to form fibers in red blood cells (RBCs), causing the cells to adopt "sickled" shapes. Using small molecules to increase the affinity of Hb for oxygen is a potential approach to treating sickle cell disease, because oxygenated Hb interferes with the polymerization of deoxyHbS. We have identified a triazole disulfide compound (4,4'-di(1,2,3-triazolyl)disulfide, designated TD-3), which increases the affinity of Hb for oxygen. The crystal structures of carboxy- and deoxy-forms of human adult Hb (HbA), each complexed with TD-3, revealed that one molecule of the monomeric thiol form of TD-3 (5-mercapto-1H-1,2,3-triazole, designated MT-3) forms a disulfide bond with β-Cys93, which inhibits the salt-bridge formation between β-Asp94 and β-His146. This inhibition of salt bridge formation stabilizes the R-state and destabilizes the T-state of Hb, resulting in reduced magnitude of the Bohr effect and increased affinity of Hb for oxygen. Intravenous administration of TD-3 (100 mg/kg) to C57BL/6 mice increased the affinity of murine Hb for oxygen, and the mice did not appear to be adversely affected by the drug. TD-3 reduced in vitro hypoxia-induced sickling of human sickle RBCs. The percentage of sickled RBCs and the P50 of human SS RBCs by TD-3 were inversely correlated with the fraction of Hb modified by TD-3. Our study shows that TD-3, and possibly other triazole disulfide compounds that bind to Hb β-Cys93, may provide new treatment options for patients with sickle cell disease.
Keywords: Bohr effect; P50; disulfide compound; hemoglobin; oxygen binding affinity; red blood cells; sickle cell disease; sickle hemoglobin.
Conflict of interest statement
The authors declare the following competing financial interest(s): The General Hospital Cooperation has filed a patent related to TD-1 and TD-3. The atomic coordinates and structure factor files have been submitted to the Protein Data Bank under an accession code for COHbA in a complex with TD-3 (PDB ID: 6BWU) and deoxyHbA in a complex with TD-3 (PDB ID: 6BWP).
Figures

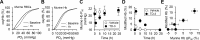

Similar articles
-
Identification of a small molecule that increases hemoglobin oxygen affinity and reduces SS erythrocyte sickling.ACS Chem Biol. 2014 Oct 17;9(10):2318-25. doi: 10.1021/cb500230b. Epub 2014 Aug 11. ACS Chem Biol. 2014. PMID: 25061917 Free PMC article.
-
Targeting βCys93 in hemoglobin S with an antisickling agent possessing dual allosteric and antioxidant effects.Metallomics. 2017 Sep 20;9(9):1260-1270. doi: 10.1039/c7mt00104e. Metallomics. 2017. PMID: 28770911 Free PMC article.
-
GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease.Br J Haematol. 2016 Oct;175(1):141-53. doi: 10.1111/bjh.14214. Epub 2016 Jul 5. Br J Haematol. 2016. PMID: 27378309
-
New developments in anti-sickling agents: can drugs directly prevent the polymerization of sickle haemoglobin in vivo?Br J Haematol. 2016 Oct;175(1):24-30. doi: 10.1111/bjh.14264. Epub 2016 Sep 8. Br J Haematol. 2016. PMID: 27605087 Free PMC article. Review.
-
Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin.Hematol Oncol Clin North Am. 2014 Apr;28(2):217-31. doi: 10.1016/j.hoc.2013.11.001. Epub 2014 Jan 21. Hematol Oncol Clin North Am. 2014. PMID: 24589263 Free PMC article. Review.
Cited by
-
Modulating hemoglobin allostery for treatment of sickle cell disease: current progress and intellectual property.Expert Opin Ther Pat. 2022 Feb;32(2):115-130. doi: 10.1080/13543776.2022.1994945. Epub 2021 Nov 1. Expert Opin Ther Pat. 2022. PMID: 34657559 Free PMC article. Review.
-
Antisickling Drugs Targeting βCys93 Reduce Iron Oxidation and Oxidative Changes in Sickle Cell Hemoglobin.Front Physiol. 2019 Jul 24;10:931. doi: 10.3389/fphys.2019.00931. eCollection 2019. Front Physiol. 2019. PMID: 31396101 Free PMC article.
-
Targeted modification of furan-2-carboxaldehydes into Michael acceptor analogs yielded long-acting hemoglobin modulators with dual antisickling activities.Chem Biol Drug Des. 2024 Jan;103(1):e14371. doi: 10.1111/cbdd.14371. Epub 2023 Oct 5. Chem Biol Drug Des. 2024. PMID: 37798397 Free PMC article.
-
Resveratrol, a New Allosteric Effector of Hemoglobin, Enhances Oxygen Supply Efficiency and Improves Adaption to Acute Severe Hypoxia.Molecules. 2023 Feb 22;28(5):2050. doi: 10.3390/molecules28052050. Molecules. 2023. PMID: 36903296 Free PMC article.
-
Hemoglobin: Structure, Function and Allostery.Subcell Biochem. 2020;94:345-382. doi: 10.1007/978-3-030-41769-7_14. Subcell Biochem. 2020. PMID: 32189307 Free PMC article. Review.
References
-
- Center for Disease Control and Prevention. https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed on Jan 17, 2018).
-
- Steinberg M. H.; McCarthy W. F.; Castro O.; Ballas S. K.; Armstrong F. D.; Smith W.; Ataga K.; Swerdlow P.; Kutlar A.; DeCastro L.; Waclawiw M. A. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell, A.; Follow-Up, M. S. H. P., The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am. J. Hematol. 2010, 85, 403–408. 10.1002/ajh.21699. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

