Molecular Signatures of Regression of the Canine Transmissible Venereal Tumor
- PMID: 29634949
- PMCID: PMC5896242
- DOI: 10.1016/j.ccell.2018.03.003
Molecular Signatures of Regression of the Canine Transmissible Venereal Tumor
Abstract
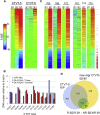
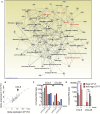
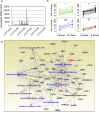
The canine transmissible venereal tumor (CTVT) is a clonally transmissible cancer that regresses spontaneously or after treatment with vincristine, but we know little about the regression mechanisms. We performed global transcriptional, methylation, and functional pathway analyses on serial biopsies of vincristine-treated CTVTs and found that regression occurs in sequential steps; activation of the innate immune system and host epithelial tissue remodeling followed by immune infiltration of the tumor, arrest in the cell cycle, and repair of tissue damage. We identified CCL5 as a possible driver of CTVT regression. Changes in gene expression are associated with methylation changes at specific intragenic sites. Our results underscore the critical role of host innate immunity in triggering cancer regression.
Keywords: CCL5; cancer; dog; epithelial; innate immunity; melanoma; methylation; regression; transmissible; vincristine.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Mechanistic Insights into Transmissible Cancers of Mammals.Cancer Cell. 2018 Apr 9;33(4):543-544. doi: 10.1016/j.ccell.2018.03.019. Cancer Cell. 2018. PMID: 29634941
References
-
- Belov K. Contagious cancer: lessons from the devil and the dog. Bioessays. 2012;34:285–292. - PubMed
-
- Bird A.P. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. - PubMed
-
- Blaine D.P. Fourth Edition. T. Boosey; 1810. A Domestic Treatise on the Diseases of Horses and Dogs.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

