Hrp48 and eIF3d contribute to msl-2 mRNA translational repression
- PMID: 29635389
- PMCID: PMC5934621
- DOI: 10.1093/nar/gky246
Hrp48 and eIF3d contribute to msl-2 mRNA translational repression
Abstract
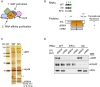
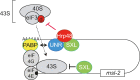
Translational repression of msl-2 mRNA in females of Drosophila melanogaster is an essential step in the regulation of X-chromosome dosage compensation. Repression is orchestrated by Sex-lethal (SXL), which binds to both untranslated regions (UTRs) of msl-2 and inhibits translation initiation by poorly understood mechanisms. Here we identify Hrp48 as a SXL co-factor. Hrp48 binds to the 3' UTR of msl-2 and is required for optimal repression by SXL. Hrp48 interacts with eIF3d, a subunit of the eIF3 translation initiation complex. Reporter and RNA chromatography assays showed that eIF3d binds to msl-2 5' UTR, and is required for efficient translation and translational repression of msl-2 mRNA. In line with these results, eIF3d depletion -but not depletion of other eIF3 subunits- de-represses msl-2 expression in female flies. These data are consistent with a model where Hrp48 inhibits msl-2 translation by targeting eIF3d. Our results uncover an important step in the mechanism of msl-2 translation regulation, and illustrate how general translation initiation factors can be co-opted by RNA binding proteins to achieve mRNA-specific control.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

