Inverse correlation between vascular endothelial growth factor back-filtration and capillary filtration pressures
- PMID: 29635428
- PMCID: PMC6113640
- DOI: 10.1093/ndt/gfy057
Inverse correlation between vascular endothelial growth factor back-filtration and capillary filtration pressures
Abstract
Background: Vascular endothelial growth factor A (VEGF) is an essential growth factor during glomerular development and postnatal homeostasis. VEGF is secreted in high amounts by podocytes into the primary urine, back-filtered across the glomerular capillary wall to act on endothelial cells. So far it has been assumed that VEGF back-filtration is driven at a constant rate exclusively by diffusion.
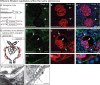
Methods: In the present work, glomerular VEGF back-filtration was investigated in vivo using a novel extended model based on endothelial fenestrations as surrogate marker for local VEGF concentrations. Single nephron glomerular filtration rate (SNGFR) and/or local filtration flux were manipulated by partial renal mass ablation, tubular ablation, and in transgenic mouse models of systemic or podocytic VEGF overexpression or reduction.
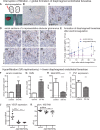
Results: Our study shows positive correlations between VEGF back-filtration and SNGFR as well as effective filtration rate under physiological conditions along individual glomerular capillaries in rodents and humans.
Conclusion: Our results suggest that an additional force drives VEGF back-filtration, potentially regulated by SNGFR.
Figures




References
-
- Jin J, Sison K, Li C. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 2012; 151: 384–399 - PubMed
-
- Haraldsson B, Barisoni L, Quaggin SE.. Reply to: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008; 359: 205–207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

