Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue
- PMID: 29635626
- PMCID: PMC5972609
- DOI: 10.1093/humrep/dey072
Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue
Abstract
Study question: Are exosomal microRNAs (miRNAs) in seminal plasma (SP) useful as markers of the origin of azoospermia and the presence of sperm in the testis?
Summary answer: Our study demonstrated the potential of several miRNAs contained in small extracellular vesicles (sEVs) of seminal fluid as sensitive and specific biomarkers for selecting those azoospermic individuals with real chances of obtaining spermatozoa from the testicular biopsy.
What is known already: There are no precise non-invasive diagnostic methods for classifying the origin of the sperm defects in semen and the spermatogenic reserve of the testis in those infertile men with a total absence of sperm in the ejaculate (azoospermia). The diagnosis of such individuals is often based on the practice of biopsies. In this context it is reasonable to study the presence of organ-specific markers in human semen that contains fluid from the testis and the male reproductive glands, which could help in the diagnosis and prognosis of male infertility. Additionally, seminal fluid contains high concentrations of sEVs that are morphologically and molecularly consistent with exosomes, which originate from multiple cellular sources in the male reproductive tract.
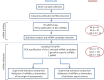
Study design, size, duration: A case and control prospective study was performed. This study compares the miRNA content of exosomes in semen samples obtained from nine normozoospermic fertile individuals (control group), 14 infertile men diagnosed with azoospermia due to spermatogenic failure, and 13 individuals with obstructive azoospermia and conserved spermatogenesis. Additionally, three severe oligozoospermic individuals (<5 × 106 sperm/ml) were included in the study.
Participants/materials, setting, methods: A differential high-throughput miRNA profiling analysis using miRNA quantitative PCR panels was performed in SP exosomes from azoospermic patients and fertile individuals.
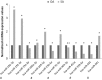
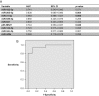
Main results and the role of chance: A total of 623 miRNAs were included in the miRNA profiling stage of the study. A total of 397 miRNAs (63.7%) were consistently detected in samples from all groups and statistically analysed, which revealed altered patterns of miRNA expression in infertile patients. We focused on the miRNAs that were differentially expressed between azoospermia as a result of an obstruction in the genital tract (i.e. having conserved spermatogenesis) and azoospermia caused by spermatogenic failure, and described, in a miRNA validation stage of the study, the expression values of one miRNA (miR-31-5p) in exosomes from semen as a predictive biomarker test for the origin of azoospermia with high sensitivity and specificity (>90%). The efficacy of the predictive test was even better when the blood FSH values were included in the analysis. Furthermore a model that included miR-539-5p and miR-941 expression values is also described as being useful for predicting the presence of residual spermatogenesis in individuals with severe spermatogenic disorders with diagnostic accuracy.
Limitations, reasons for caution: Further studies, with an independent second population involving a larger number of samples, are needed to confirm our findings.
Wider implications of the findings: Our findings contribute to the search for the most valuable genetic markers that are potentially useful as tools for predicting the presence of testicular sperm in azoospermic individuals.
Study funding/competing interest(s): This work was financially supported by grants from the Fondo de Investigaciones Sanitarias/Fondo Europeo de Desarrollo Regional "Una manera de hacer Europa" (FIS/FEDER) [Grant number PI15/00153], the Generalitat de Catalunya [Grant number 2014SGR5412]. S.L. is sponsored by the Researchers Stabilization Program (ISCIII/Generalitat de Catalunya) from the Spanish National Health System [CES09/020].
Figures
References
-
- Aalberts M, Sostaric E, Wubbolts R, Wauben MW, Nolte-‘t Hoen EN, Gadella BM, Stout TA, Stoorvogel W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta 2013;1834:2326–2335. - PubMed
-
- Abu-Halima M, Ludwig N, Hart M, Leidinger P, Backes C, Keller A, Hammadeh M, Meese E. Altered micro-ribonucleic acid expression profiles of extracellular microvesicles in the seminal plasma of patients with oligoasthenozoospermia. Fertil Steril 2016;106:1061–1069.e1063. - PubMed
-
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL et al. . Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011;108:5003–5008. - PMC - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

