Pectoralis muscle area and mortality in smokers without airflow obstruction
- PMID: 29636050
- PMCID: PMC5894181
- DOI: 10.1186/s12931-018-0771-6
Pectoralis muscle area and mortality in smokers without airflow obstruction
Abstract
Background: Low muscle mass is associated with increased mortality in the general population but its prognostic value in at-risk smokers, those without expiratory airflow obstruction, is unknown. We aimed to test the hypothesis that reduced muscle mass is associated with increased mortality in at-risk smokers.
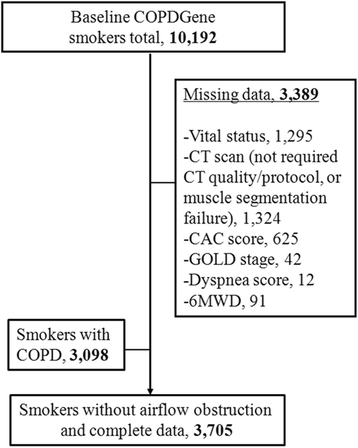
Methods: Measures of both pectoralis and paravertebral erector spinae muscle cross-sectional area (PMA and PVMA, respectively) as well as emphysema on chest computed tomography (CT) scans were performed in 3705 current and former at-risk smokers (≥10 pack-years) aged 45-80 years enrolled into the COPDGene Study between 2008 and 2013. Vital status was ascertained through death certificate. The association between low muscle mass and mortality was assessed using Cox regression analysis.
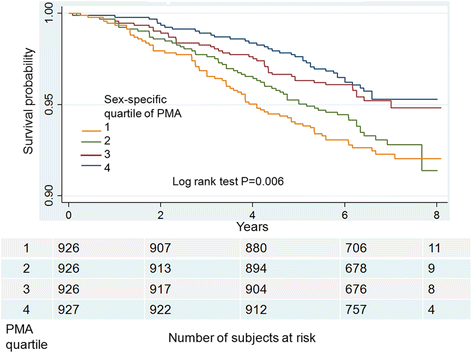
Results: During a median of 6.5 years of follow-up, 212 (5.7%) at-risk smokers died. At-risk smokers in the lowest (vs. highest) sex-specific quartile of PMA but not PVMA had 84% higher risk of death in adjusted models for demographics, smoking, dyspnea, comorbidities, exercise capacity, lung function, emphysema on CT, and coronary artery calcium content (hazard ratio [HR] 1.85 95% Confidence interval [1.14-3.00] P = 0.01). Results were consistent when the PMA index (PMA/height2) was used instead of quartiles. The association between PMA and death was modified by smoking status (P = 0.04). Current smokers had a significantly increased risk of death (lowest vs. highest PMA quartile, HR 2.25 [1.25-4.03] P = 0.007) while former smokers did not.
Conclusions: Low muscle mass as measured on chest CT scans is associated with increased mortality in current smokers without airflow obstruction.
Trial registration: NCT00608764.
Keywords: CT; Paravertebral muscle mass; Pectoralis muscle mass; Smoking.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by institutional review board of each of the 21 participating and all participants gave written informed consent. The Partners HealthCare Research Committee approved the current analysis (2007P-000554).
Consent for publication
Not applicable.
Competing interests
Drs. AD, CM, RH, MM, JR, MH, RB, BM, ER, ES, JC, AB, GK, JH, RSJ, and GW and Mr. TY have no conflict of interest related to this work. Dr. AD has received speaker fees from Novartis Inc. unrelated to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical