Allopregnanolone alters follicular and luteal dynamics during the estrous cycle
- PMID: 29636114
- PMCID: PMC5894215
- DOI: 10.1186/s12958-018-0353-y
Allopregnanolone alters follicular and luteal dynamics during the estrous cycle
Abstract
Background: Allopregnanolone is a neurosteroid synthesized in the central nervous system independently of steroidogenic glands; it influences sexual behavior and anxiety. The aim of this work is to evaluate the indirect effect of a single pharmacological dose of allopregnanolone on important processes related to normal ovarian function, such as folliculogenesis, angiogenesis and luteolysis, and to study the corresponding changes in endocrine profile and enzymatic activity over 4 days of the rat estrous cycle. We test the hypothesis that allopregnanolone may trigger hypothalamus - hypophysis - ovarian axis dysregulation and cause ovarian failure which affects the next estrous cycle stages.
Methods: Allopregnanolone was injected during the proestrous morning and then, the animals were sacrificed at each stage of the estrous cycle. Ovarian sections were processed to determine the number and diameter of different ovarian structures. Cleaved caspase 3, proliferating cell nuclear antigen, α-actin and Von Willebrand factor expressions were evaluated by immunohistochemistry. Luteinizing hormone, prolactin, estrogen and progesterone serum levels were measured by radioimmunoassay. The enzymatic activities of 3β-hydroxysteroid dehydrogenase, 3α-hydroxysteroid oxidoreductase and 20α-hydroxysteroid dehydrogenase were determined by spectrophotometric assays. Two-way ANOVA followed by Bonferroni was performed to determine statistical differences between control and treated groups along the four stages of the cycle.
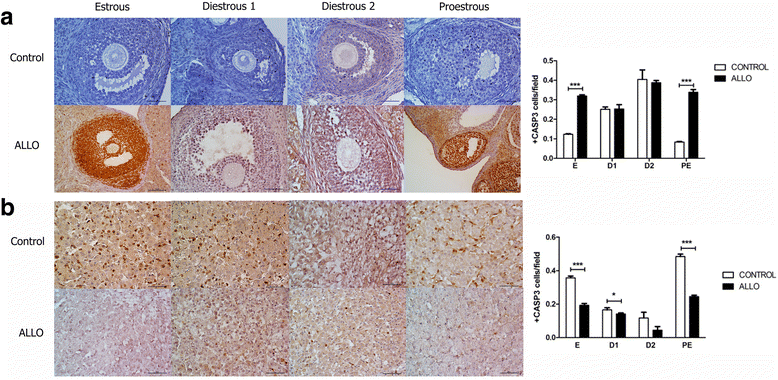
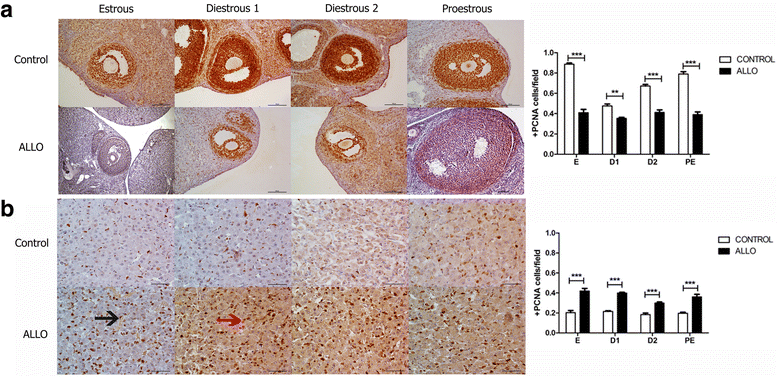
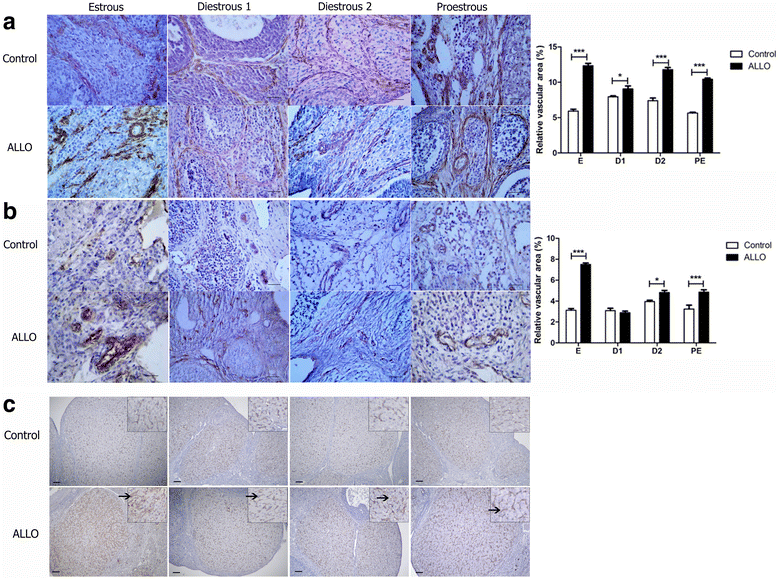
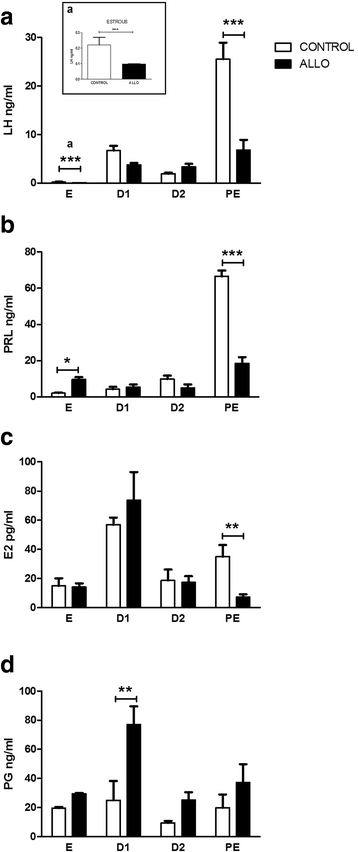
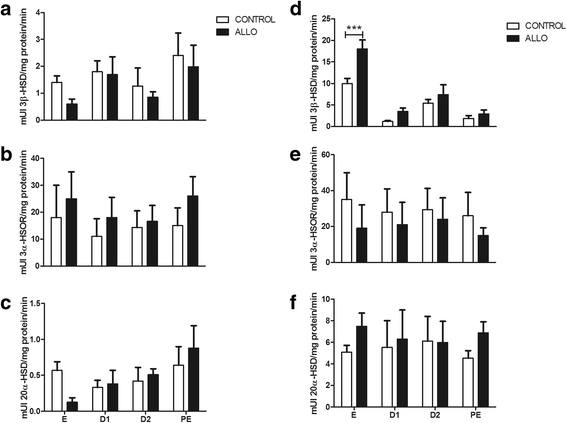
Results: The results indicate that allopregnanolone allopregnanolone decreased the number of developing follicles, while atretic follicles and cysts increased with no effects on normal cyclicity. Some cysts in treated ovaries showed morphological characteristics similar to luteinized unruptured follicles. The apoptosis/proliferation balance increased in follicles from treated rats. The endocrine profile was altered at different stages of the estrous cycle of treated rats. The angiogenic markers expression increased in treated ovaries. As regards corpora lutea, the apoptosis/proliferation balance and 20α-hydroxysteroid dehydrogenase enzymatic activity decreased significantly. Progesterone levels and 3β-hydroxysteroid dehydrogenase enzymatic activity increased in treated rats. These data suggest that allopregnanolone interferes with steroidogenesis and folliculogenesis at different stages of the cycle.
Conclusion: Allopregnanolone interferes with corpora lutea regression, which might indicate that this neurosteroid exerts a protective role over the luteal cells and prevents them from luteolysis. Allopregnanolone plays an important role in the ovarian pathophysiology.
Keywords: Allopregnanolone; Angiogenesis; Apoptosis; Corpora lutea; Folliculogenesis; Luteolysis; Steroidogenesis.
Conflict of interest statement
Ethics approval
All protocols were previously approved by the Institutional Committee for Care and Use of Experimental Animals (CICUAL N° 141021) and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the National Research Council (National Academies, U.S.A., 8th Edition, 2011).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A single dose of allopregnanolone affects the ovarian morphology and steroidogenesis.Reproduction. 2016 Oct 24:REP-16-0463. doi: 10.1530/REP-16-0463. Online ahead of print. Reproduction. 2016. PMID: 27777323
-
Allopregnanolone alters the luteinizing hormone, prolactin, and progesterone serum levels interfering with the regression and apoptosis in rat corpus luteum.Horm Metab Res. 2012 Jul;44(8):632-8. doi: 10.1055/s-0032-1314834. Epub 2012 Jun 6. Horm Metab Res. 2012. PMID: 22674474
-
An enzymologic study of corpora lutea in early pregnant rats treated with abortifacient agents.Fertil Steril. 1976 Aug;27(8):980-6. doi: 10.1016/s0015-0282(16)42018-2. Fertil Steril. 1976. PMID: 955139
-
The newly formed corpora lutea of normal cycling rats exhibit drastic changes in steroidogenic and luteolytic gene expressions.Exp Toxicol Pathol. 2012 Nov;64(7-8):775-82. doi: 10.1016/j.etp.2011.01.015. Epub 2011 Feb 22. Exp Toxicol Pathol. 2012. PMID: 21345661
-
Neonatal superior ovarian nerve transection disturbs the cyclic activity of the female rats.J Steroid Biochem Mol Biol. 2002 Sep;82(1):75-82. doi: 10.1016/s0960-0760(02)00149-8. J Steroid Biochem Mol Biol. 2002. PMID: 12429141
Cited by
-
Validation of an ELISA kit to measure allopregnanolone in human and equine hair.J Vet Diagn Invest. 2023 Jul;35(4):354-358. doi: 10.1177/10406387231171045. Epub 2023 Apr 28. J Vet Diagn Invest. 2023. PMID: 37114774 Free PMC article.
-
Local effect of allopregnanolone in rat ovarian steroidogenesis, follicular and corpora lutea development.Sci Rep. 2024 Mar 16;14(1):6402. doi: 10.1038/s41598-024-57102-1. Sci Rep. 2024. PMID: 38493224 Free PMC article.
References
-
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjövall J, et al. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. - DOI - PubMed
-
- Baulieu EE. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

