Mobile Phone Support for Diabetes Self-Care Among Diverse Adults: Protocol for a Three-Arm Randomized Controlled Trial
- PMID: 29636319
- PMCID: PMC5915673
- DOI: 10.2196/resprot.9443
Mobile Phone Support for Diabetes Self-Care Among Diverse Adults: Protocol for a Three-Arm Randomized Controlled Trial
Abstract
Background: Nonadherence to self-care is common among patients with type 2 diabetes (T2D) and often leads to severe complications. Moreover, patients with T2D who have low socioeconomic status and are racial/ethnic minorities disproportionately experience barriers to adherence and poor outcomes. Basic phone technology (text messages and phone calls) provides a practical medium for delivering content to address patients' barriers to adherence; however, trials are needed to explore long-term and sustainable effects of mobile phone interventions among diverse patients.
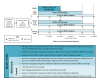
Objective: The aim of this study is to evaluate the effects of mobile phone-based diabetes support interventions on self-care and hemoglobin A1c (HbA1c) among adults with T2D using a 3-arm, 15-month randomized controlled trial with a Type 1 hybrid effectiveness-implementation approach. The intervention arms are (1) Rapid Encouragement/Education And Communications for Health (REACH) and (2) REACH + Family-focused Add-on for Motivating Self-care (FAMS).
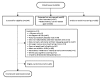
Methods: We recruited primary care patients with T2D (N=512) from Federally Qualified Health Centers and an academic medical center, prioritizing recruitment of publicly insured and minority patients from the latter. Eligible patients were prescribed daily diabetes medication and owned a cell phone with text messaging capability. We excluded patients whose most recent HbA1c result within 12 months was <6.8% to support detection of intervention effects on HbA1c. Participants were randomly assigned to REACH only, REACH + FAMS, or the control condition. REACH provides text messages tailored to address patient-specific barriers to medication adherence based on the Information-Motivation-Behavioral skills model, whereas FAMS provides monthly phone coaching with related text message content focused on family and friend barriers to diet and exercise adherence. We collect HbA1c and self-reported survey data at baseline and at 3, 6, and 12 months, and again at 15 months to assess sustained changes. We will use generalized estimating equation models to test the effects of REACH (either intervention arm) on HbA1c relative to the control group, the potential additive effects of FAMS, and effects of either intervention on adherence to self-care behaviors and diabetes self-efficacy.
Results: The trial is ongoing; recruitment closed December 2017. We plan to perform analyses on 6-month outcomes for FAMS in July 2018, and project to have 15-month data for REACH analyses in April 2019.
Conclusions: Our study will be one of the first to evaluate a long-term, theory-based text messaging intervention to promote self-care adherence among racially/ethnically and socioeconomically diverse adults with T2D. Moreover, our study will assess the feasibility of a family-focused intervention delivered via mobile phones and compare the effects of text messaging alone versus text messaging plus phone coaching. Findings will advance our understanding of how interventions delivered by phone can benefit diverse patients with chronic conditions.
Trial registration: ClinicalTrials.gov NCT02409329; https://clinicaltrials.gov/ct2/show/NCT02409329 (Archived by WebCite at http://www.webcitation.org/6yHkg9SSl); NCT02481596; https://clinicaltrials.gov/ct2/show/NCT02481596 (Archived by WebCite at http://www.webcitation.org/6yHkj9XD4).
Keywords: glycated hemoglobin; medication adherence; mobile health; self-care; text messaging; type 2 diabetes.
©Lyndsay A Nelson, Kenneth A Wallston, Sunil Kripalani, Robert A Greevy Jr, Tom A Elasy, Erin M Bergner, Chad K Gentry, Lindsay S Mayberry. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 10.04.2018.
Conflict of interest statement
Conflicts of Interest: KW is a member of the Advisory Board for EdLogics, Inc.
Figures
References
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017 Jun;128:40–50. doi: 10.1016/j.diabres.2017.03.024.S0168-8227(17)30375-3 - DOI - PubMed
-
- Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de BIH. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016 Aug 09;316(6):602–10. doi: 10.1001/jama.2016.10924. http://europepmc.org/abstract/MED/27532915 2542635 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. [2017-03-02]. National diabetes statistics report, 2017 https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-stat... .
-
- National Institute of Diabetes Digestive Kidney Diseases. [2017-03-02]. Diabetes, heart disease, and stroke https://www.niddk.nih.gov/health-information/diabetes/overview/preventin... .
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

