Resistance Mechanisms to Targeted Therapies in ROS1+ and ALK+ Non-small Cell Lung Cancer
- PMID: 29636358
- PMCID: PMC6050099
- DOI: 10.1158/1078-0432.CCR-17-2452
Resistance Mechanisms to Targeted Therapies in ROS1+ and ALK+ Non-small Cell Lung Cancer
Abstract
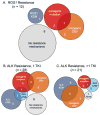
Purpose: Despite initial benefit from tyrosine kinase inhibitors (TKIs), patients with advanced non-small cell lung cancer (NSCLC) harboring ALK (ALK+) and ROS1 (ROS1+) gene fusions ultimately progress. Here, we report on the potential resistance mechanisms in a series of patients with ALK+ and ROS1+ NSCLC progressing on different types and/or lines of ROS1/ALK-targeted therapy.Experimental Design: We used a combination of next-generation sequencing (NGS), multiplex mutation assay, direct DNA sequencing, RT-PCR, and FISH to identify fusion variants/partners and copy-number gain (CNG), kinase domain mutations (KDM), and copy-number variations (CNVs) in other cancer-related genes. We performed testing on 12 ROS1+ and 43 ALK+ patients.Results: One of 12 ROS1+ (8%) and 15 of 43 (35%) ALK + patients harbored KDM. In the ROS1+ cohort, we identified KIT and β-catenin mutations and HER2-mediated bypass signaling as non-ROS1-dominant resistance mechanisms. In the ALK+ cohort, we identified a novel NRG1 gene fusion, a RET fusion, 2 EGFR, and 3 KRAS mutations, as well as mutations in IDH1, RIT1, NOTCH, and NF1 In addition, we identified CNV in multiple proto-oncogenes genes including PDGFRA, KIT, KDR, GNAS, K/HRAS, RET, NTRK1, MAP2K1, and others.Conclusions: We identified a putative TKI resistance mechanism in six of 12 (50%) ROS1 + patients and 37 of 43 (86%) ALK+ patients. Our data suggest that a focus on KDMs will miss most resistance mechanisms; broader gene testing strategies and functional validation is warranted to devise new therapeutic strategies for drug resistance. Clin Cancer Res; 24(14); 3334-47. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflicts of Interest.
CEM- honoraria for Takeda, Guardant Health, DRC- honoraria from Takeda; RD – Pfizer speaker’s bureau and advisory board, Novartis, Roche, Ignyta – advisory board; RCD – AstraZeneca – advisory board, Guardant Health – honoraria, Pfizer – honoraria, Ignyta – advisory board and sponsored research grant, Takeda – advisory board, Spectrum Pharmaceuticals – advisory board, Abbott Molecular – remuneration for patent license, Rain Therapeutics – stock owner; PAB – consultant for Genentech and Pfizer; ATL – Abbott Molecular – remuneration for patent license; DTM, ATL, KG, KJ, LS, AD, AEB, KDD, MV-G, WTP, DLA - declare no potential conflicts of interest;
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

