Cardiac c-Kit Biology Revealed by Inducible Transgenesis
- PMID: 29636378
- PMCID: PMC6192707
- DOI: 10.1161/CIRCRESAHA.117.311828
Cardiac c-Kit Biology Revealed by Inducible Transgenesis
Abstract
Rationale: Biological significance of c-Kit as a cardiac stem cell marker and role(s) of c-Kit+ cells in myocardial development or response to pathological injury remain unresolved because of varied and discrepant findings. Alternative experimental models are required to contextualize and reconcile discordant published observations of cardiac c-Kit myocardial biology and provide meaningful insights regarding clinical relevance of c-Kit signaling for translational cell therapy.
Objective: The main objectives of this study are as follows: demonstrating c-Kit myocardial biology through combined studies of both human and murine cardiac cells; advancing understanding of c-Kit myocardial biology through creation and characterization of a novel, inducible transgenic c-Kit reporter mouse model that overcomes limitations inherent to knock-in reporter models; and providing perspective to reconcile disparate viewpoints on c-Kit biology in the myocardium.
Methods and results: In vitro studies confirm a critical role for c-Kit signaling in both cardiomyocytes and cardiac stem cells. Activation of c-Kit receptor promotes cell survival and proliferation in stem cells and cardiomyocytes of either human or murine origin. For creation of the mouse model, the cloned mouse c-Kit promoter drives Histone2B-EGFP (enhanced green fluorescent protein; H2BEGFP) expression in a doxycycline-inducible transgenic reporter line. The combination of c-Kit transgenesis coupled to H2BEGFP readout provides sensitive, specific, inducible, and persistent tracking of c-Kit promoter activation. Tagging efficiency for EGFP+/c-Kit+ cells is similar between our transgenic versus a c-Kit knock-in mouse line, but frequency of c-Kit+ cells in cardiac tissue from the knock-in model is 55% lower than that from our transgenic line. The c-Kit transgenic reporter model reveals intimate association of c-Kit expression with adult myocardial biology. Both cardiac stem cells and a subpopulation of cardiomyocytes express c-Kit in uninjured adult heart, upregulating c-Kit expression in response to pathological stress.
Conclusions: c-Kit myocardial biology is more complex and varied than previously appreciated or documented, demonstrating validity in multiple points of coexisting yet heretofore seemingly irreconcilable published findings.
Keywords: c-Kit protein; myocardium; myocytes, cardiac; signal transduction; stem cell.
© 2018 American Heart Association, Inc.
Figures
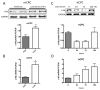





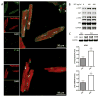


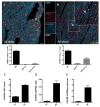
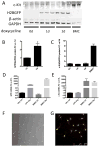


Comment in
-
Reassessment of c-Kit in Cardiac Cells: A Complex Interplay Between Expression, Fate, and Function.Circ Res. 2018 Jun 22;123(1):9-11. doi: 10.1161/CIRCRESAHA.118.313215. Circ Res. 2018. PMID: 29929968 Free PMC article. No abstract available.
References
-
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10344–10349. - PMC - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1808–1813. - PMC - PubMed
-
- Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells and Development. 2010;19:105–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL122525/HL/NHLBI NIH HHS/United States
- U54 CA132384/CA/NCI NIH HHS/United States
- R01 HL135661/HL/NHLBI NIH HHS/United States
- R01 HL105759/HL/NHLBI NIH HHS/United States
- F32 HL131299/HL/NHLBI NIH HHS/United States
- R00 HL112852/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- F32 HL136196/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- U54 CA132379/CA/NCI NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- R01 HL130072/HL/NHLBI NIH HHS/United States
- P01 HL085577/HL/NHLBI NIH HHS/United States
- R01 HL117163/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

