Extracellular Electron Transfer Powers Enterococcus faecalis Biofilm Metabolism
- PMID: 29636430
- PMCID: PMC5893876
- DOI: 10.1128/mBio.00626-17
Extracellular Electron Transfer Powers Enterococcus faecalis Biofilm Metabolism
Erratum in
-
Correction for Keogh et al., "Extracellular Electron Transfer Powers Enterococcus faecalis Biofilm Metabolism".mBio. 2019 Jun 18;10(3):e01080-19. doi: 10.1128/mBio.01080-19. mBio. 2019. PMID: 31213555 Free PMC article. No abstract available.
Abstract
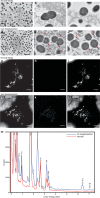
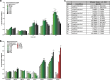
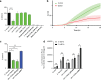
Enterococci are important human commensals and significant opportunistic pathogens. Biofilm-related enterococcal infections, such as endocarditis, urinary tract infections, wound and surgical site infections, and medical device-associated infections, often become chronic upon the formation of biofilm. The biofilm matrix establishes properties that distinguish this state from free-living bacterial cells and increase tolerance to antimicrobial interventions. The metabolic versatility of the enterococci is reflected in the diversity and complexity of environments and communities in which they thrive. Understanding metabolic factors governing colonization and persistence in different host niches can reveal factors influencing the transition to biofilm pathogenicity. Here, we report a form of iron-dependent metabolism for Enterococcus faecalis where, in the absence of heme, extracellular electron transfer (EET) and increased ATP production augment biofilm growth. We observe alterations in biofilm matrix depth and composition during iron-augmented biofilm growth. We show that the ldh gene encoding l-lactate dehydrogenase is required for iron-augmented energy production and biofilm formation and promotes EET.IMPORTANCE Bacterial metabolic versatility can often influence the outcome of host-pathogen interactions, yet causes of metabolic shifts are difficult to resolve. The bacterial biofilm matrix provides the structural and functional support that distinguishes this state from free-living bacterial cells. Here, we show that the biofilm matrix can immobilize iron, providing access to this growth-promoting resource which is otherwise inaccessible in the planktonic state. Our data show that in the absence of heme, Enterococcus faecalis l-lactate dehydrogenase promotes EET and uses matrix-associated iron to carry out EET. Therefore, the presence of iron within the biofilm matrix leads to enhanced biofilm growth.
Keywords: Enterococcus faecalis; biofilm; extracellular electron transfer; iron; metabolism.
Copyright © 2018 Keogh et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
