Study of mucin turnover in the small intestine by in vivo labeling
- PMID: 29636525
- PMCID: PMC5893601
- DOI: 10.1038/s41598-018-24148-x
Study of mucin turnover in the small intestine by in vivo labeling
Abstract
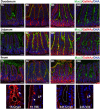
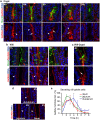
Mucins are highly glycosylated proteins which protect the epithelium. In the small intestine, the goblet cell-secreted Muc2 mucin constitutes the main component of the loose mucus layer that traps luminal material. The transmembrane mucin Muc17 forms part of the carbohydrate-rich glycocalyx covering intestinal epithelial cells. Our study aimed at investigating the turnover of these mucins in the small intestine by using in vivo labeling of O-glycans with N-azidoacetylgalactosamine. Mice were injected intraperitoneally and sacrificed every hour up to 12 hours and at 24 hours. Samples were fixed with preservation of the mucus layer and stained for Muc2 and Muc17. Turnover of Muc2 was slower in goblet cells of the crypts compared to goblet cells along the villi. Muc17 showed stable expression over time at the plasma membrane on villi tips, in crypts and at crypt openings. In conclusion, we have identified different subtypes of goblet cells based on their rate of mucin biosynthesis and secretion. In order to protect the intestinal epithelium from chemical and bacterial hazards, fast and frequent renewal of the secreted mucus layer in the villi area is combined with massive secretion of stored Muc2 from goblet cells in the upper crypt.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ermund A, Schutte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol Gastrointest. Liver Physiol. 2013;305:G341–G347. doi: 10.1152/ajpgi.00046.2013. - DOI - PMC - PubMed
-
- Rodriguez-Pineiro AM, et al. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol Gastrointest. Liver Physiol. 2013;305:G348–G356. doi: 10.1152/ajpgi.00047.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

