Anticancer activity of the intraperitoneal-delivered DFP-10825, the cationic liposome-conjugated RNAi molecule targeting thymidylate synthase, on peritoneal disseminated ovarian cancer xenograft model
- PMID: 29636601
- PMCID: PMC5881279
- DOI: 10.2147/DDDT.S156635
Anticancer activity of the intraperitoneal-delivered DFP-10825, the cationic liposome-conjugated RNAi molecule targeting thymidylate synthase, on peritoneal disseminated ovarian cancer xenograft model
Abstract
Introduction: Peritoneal disseminated ovarian cancer is one of the most difficult cancers to treat with conventional anti-cancer drugs and the treatment options are very limited, although an intraperitoneal (ip) paclitaxel has shown some clinical benefit. Therefore, treatment of peritoneal disseminated ovarian cancer is a highly unmet medical need and it is urgent to develop a new ip delivered drug regulating the fast DNA synthesis.
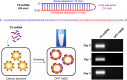
Methods: We developed a unique RNAi molecule consisting of shRNA against the thymidylate synthase (TS) and a cationic liposome (DFP-10825) and tested its antitumor activity and PK profile in peritoneally disseminated human ovarian cancer ascites models by the luciferase gene-transfected SCID mice. DFP-10825 alone, paclitaxel alone or combination with DFP-10825 and paclitaxel were administered in an ip route to the tumor-bearing mice. The TS expression level was measured by conventional RT-PCR. The anti-tumor activity and host survival benefit by DFP-10825 treatment on tumor-bearing mice were observed as resulting from the specific TS mRNA knock-down in tumors.
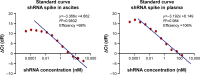
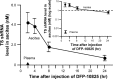
Results: DFP-10825 alone significantly suppressed the growth of SKOV3-luc tumore ascites cells and further extended the survival time of these tumor-bearing mice. Combination with the ip paclitaxel augmented the antitumor efficacy of DFP-10825 and significantly prolonged the survival time in the tumor-bearing mice. Short-hairpin RNA for TS (TS shRNA) levels derived from DFP-10825 in the ascetic fluid were maintained at a nM range across 24 hours but not detected in the plasma, suggesting that TS shRNA is relatively stable in the peritoneal cavity, to be able to exert its anti-tumor activity, but not in blood stream, indicating little or no systemic effect.
Conclusion: Collectively, the ip delivery of DFP-10825, TS shRNA conjugated with cationic liposome, shows a favorable antitumor activity without systemic adverse events via the stable localization of TS shRNA for a sufficient time and concentration in the peritoneal cavity of the peritoneally disseminated human ovarian cancer-bearing mice.
Keywords: DFP-10825; cationic liposome; intraperitoneal dissemination; ip delivery; ovarian cancer; short-hairpin RNA; thymidylate synthase.
Conflict of interest statement
Disclosure All authors are employees of Delta-Fly Pharma Inc. The authors report no other conflicts of interest in this work.
Figures




References
-
- Kitayama J. Intraperitoneal chemotherapy against peritoneal carcinomatosis: current status and future perspective. Surg Oncol. 2014;23(2):99–106. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, et al. Gynecologic Oncology Group Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21(17):3194–3200. - PubMed
-
- McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. - PubMed
-
- Armstrong DK, Bundy B, Wenzel L, et al. Gynecologic Oncology Group Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

