KIF18B promotes tumor progression through activating the Wnt/β-catenin pathway in cervical cancer
- PMID: 29636620
- PMCID: PMC5880519
- DOI: 10.2147/OTT.S157440
KIF18B promotes tumor progression through activating the Wnt/β-catenin pathway in cervical cancer
Abstract
Background: KIF18B was identified as a potential oncogene by analysis of The Cancer Genome Atlas database.
Materials and methods: We assessed KIF18B expression and explored its clinical significance in cervical cancer tissues. We have also evaluated the effects of KIF18B on cervical cancer cell proliferation, migration, and invasion both in vitro and in vivo.
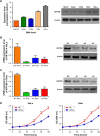
Results: Our results show that KIF18B is overexpressed in cervical cancer tissues and is associated with a large primary tumor size, an advanced FIGO stage, and an advanced tumor grade. Knockdown of KIF18B induces cell cycle G1-phase arrest and inhibits the proliferation, migration, and invasion of cervical cancer cells, whereas its overexpression promotes proliferation, migration, and invasion in these cells. Moreover, silencing of KIF18B reduces expression of CyclinD1, β-catenin, C-myc, and p-GSK3β expression.
Conclusion: These data suggest that KIF18B can serve as a novel oncogene that promotes the tumorigenicity of cervical cancer cells by activating Wnt/β-catenin signaling pathway.
Keywords: CyclinD1; KIF18B; Wnt/β-catenin signaling pathway; cervical cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








Similar articles
-
KIF18B promotes breast cancer cell proliferation, migration and invasion by targeting TRIP13 and activating the Wnt/β-catenin signaling pathway.Oncol Lett. 2022 Apr;23(4):112. doi: 10.3892/ol.2022.13232. Epub 2022 Feb 8. Oncol Lett. 2022. PMID: 35251343 Free PMC article.
-
KIF18B promotes hepatocellular carcinoma progression through activating Wnt/β-catenin-signaling pathway.J Cell Physiol. 2020 Oct;235(10):6507-6514. doi: 10.1002/jcp.29444. Epub 2020 Feb 13. J Cell Physiol. 2020. PMID: 32052444
-
MiRNA-139-3p inhibits malignant progression in urothelial carcinoma of the bladder via targeting KIF18B and inactivating Wnt/beta-catenin pathway.Pharmacogenet Genomics. 2023 Jan 1;33(1):1-9. doi: 10.1097/FPC.0000000000000485. Epub 2022 Nov 7. Pharmacogenet Genomics. 2023. PMID: 36441170
-
KIF18B promotes tumor progression in osteosarcoma by activating β-catenin.Cancer Biol Med. 2020 May 15;17(2):371-386. doi: 10.20892/j.issn.2095-3941.2019.0452. Cancer Biol Med. 2020. PMID: 32587775 Free PMC article.
-
KIF18B: an important role in signaling pathways and a potential resistant target in tumor development.Discov Oncol. 2024 Sep 11;15(1):430. doi: 10.1007/s12672-024-01330-4. Discov Oncol. 2024. PMID: 39259333 Free PMC article. Review.
Cited by
-
Identification of Prognostic Markers and Potential Therapeutic Targets in Gastric Adenocarcinoma by Machine Learning Based on mRNAsi Index.J Oncol. 2022 Sep 30;2022:8926127. doi: 10.1155/2022/8926127. eCollection 2022. J Oncol. 2022. PMID: 36213825 Free PMC article.
-
miR-139-3p/Kinesin family member 18B axis suppresses malignant progression of gastric cancer.Bioengineered. 2022 Feb;13(2):4528-4536. doi: 10.1080/21655979.2022.2033466. Bioengineered. 2022. PMID: 35137670 Free PMC article.
-
lncRNA WT1-AS inhibits the aggressiveness of cervical cancer cell via regulating p53 expression via sponging miR-330-5p.Cancer Manag Res. 2019 Jan 11;11:651-667. doi: 10.2147/CMAR.S176525. eCollection 2019. Cancer Manag Res. 2019. PMID: 30666161 Free PMC article.
-
Nucleolar and spindle associated protein 1 promotes metastasis of cervical carcinoma cells by activating Wnt/β-catenin signaling.J Exp Clin Cancer Res. 2019 Jan 24;38(1):33. doi: 10.1186/s13046-019-1037-y. J Exp Clin Cancer Res. 2019. PMID: 30678687 Free PMC article.
-
Kinesin family member 18B regulates the proliferation and invasion of human prostate cancer cells.Cell Death Dis. 2021 Mar 22;12(4):302. doi: 10.1038/s41419-021-03582-2. Cell Death Dis. 2021. PMID: 33753726 Free PMC article.
References
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Garland SM, Cuzick J, Domingo EJ, et al. Recommendations for cervical cancer prevention in Asia Pacific. Vaccine. 2008;(Suppl 12):M89–M98. - PubMed
-
- Schiffman MH, Brinton LA. The epidemiology of cervical carcinogenesis. Cancer. 1995;76(10 Suppl):1888–1901. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

