Antibody-sandwich ELISA analysis of a novel blood biomarker of CST4 in gastrointestinal cancers
- PMID: 29636621
- PMCID: PMC5880518
- DOI: 10.2147/OTT.S149204
Antibody-sandwich ELISA analysis of a novel blood biomarker of CST4 in gastrointestinal cancers
Abstract
Background: Members of the cystatin family have increasingly been proven to be involved in several tumors, including gastric cancer (GC) and colorectal cancer (CRC). Cystatin S (CST4) was found to be upregulated at the gene expression level in GC cells, making it a potential novel biomarker for the early diagnosis of gastrointestinal cancer.
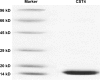
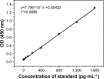
Materials and methods: Quantitative real-time polymerase chain reaction and Western blotting analysis were used to explore CST4 expression in gastrointestinal cancer tissues and cell lines. We purified CST4 recombinant protein and generated anti-CST4 monoclonal antibodies to develop an antibody-sandwich enzyme-linked immunosorbent assay (ELISA) analysis system for blood CST4 detection. The performance and clinical efficacy of the detection method were evaluated using a training set and validation set, respectively.
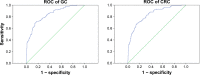
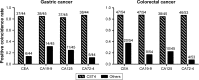
Results: According to the quantitative real-time polymerase chain reaction and Western blotting results, CST4-mRNA expression and protein expression were upregulated in gastrointestinal cancer tissues and cell lines. The ELISA detection system for CST4 showed significantly better sensitivities of 69.0% and 69.0% and specificities of 85.6% and 83.6% for GC and CRC, respectively, than other common clinical biomarkers, carcinoembryonic antigen, CA19-9, CA125, and CA72-4. Clinical verification experiments using GC and CRC validation sets also found distinguishable CST4 median concentrations (177.7 pg·mL-1 and 174.2 pg·mL-1 respectively) and high positive detection rates (72.3% and 88.4% respectively), further confirming the specificity and sensitivity of this method.
Conclusion: We validated the overexpression of CST4 in gastrointestinal cancer tissues and cell lines and developed an antibody-sandwich ELISA analysis system for blood CST4 detection, which exhibited high specificity and sensitivity. Novel blood biomarkers of CST4 have enormous potential in terms of clinical diagnostic value in GC and CRC.
Keywords: ELISA; biomarker; cystatin S; gastrointestinal cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN, 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Sung JJY, Ng EK, Lin JT, et al. Asia Pacific GI Oncology Summit Group Digestive cancer management in Asia: position statements: a report on GI oncology summit in 2011. J Gastroen Hepatol. 2012;27(9):1417–1422. - PubMed
-
- Ouyang DL, Chen JJ, Getzenberg RH, Schoen RE. Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol. 2005;100(6):1393–1403. - PubMed
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, Colorectal Cancer Study Group Fecal DNA versus fecal occult blood for colorectal cancer screening in an average-risk population. N Engl J Med. 2004;351(26):2704–2714. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

