A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis
- PMID: 29636627
- PMCID: PMC5880515
- DOI: 10.2147/JIR.S160964
A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis
Abstract
Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are dermatologic emergencies with high morbidity and mortality risk. Cyclosporine, an immunomodulatory agent, is sometimes used off-label, and its role continues to be debated. This meta-analysis aimed to provide an update of current evidence and to clarify the role of cyclosporine in SJS/TEN treatment better.
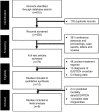
Methods: Using the keywords [cyclosporine OR cyclosporine OR ciclosporin OR CsA] AND [Steven-Johnson OR SJS OR toxic epidermal OR epidermal necrolysis OR TEN OR hypersensitivity OR dermatologic OR burns], a preliminary search on the PubMed, Ovid, Web of Science, and Google Scholar Database yielded 615 papers published in English between January1, 1960 and July 1, 2017. The inclusion criteria for this review were: 1) published retrospective or prospective study (excluding single case reports); 2) patients with clinical diagnosis of SJS or TEN; 3) trial of cyclosporine treatment; and 4) available survival/mortality data.
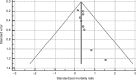
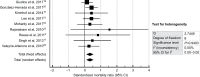
Results: A total of 12 studies, with a total of 358 SJS/TEN patients were reviewed. Two studies were excluded from the meta-analysis as they did not report SCORe of toxic epidermal necrosis/predicted mortality data; one was excluded because of possible data irregularities. Meta-analysis of nine studies revealed a significant reduction in mortality risk with cyclosporine therapy (standardized mortality ratio 0.320; 95% CI: 0.119-0.522; P=0.002). Cyclosporine was also generally well tolerated with little adverse effects or increased infection, albeit the patients tended to be critically ill. Publication bias was observed in the funnel plot and Egger test (P=0.0467).
Conclusion: Currently available evidence are predominantly open trials and retrospective studies with a significant risk of bias, perhaps owing to the rarity and life-threatening nature of the condition. Given its immunomodulatory actions, cyclosporine could be a potential treatment option for SJS/TEN in addition to best supportive measures. Further confirmation with robust randomized, controlled trials or larger case series is necessary and should be encouraged.
Keywords: CsA; SJS; TEN; cyclosporine; epidermal necrolysis; meta-analysis.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. - PubMed
-
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173–e1. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

