A Versatile New Model of Chemically Induced Chronic Colitis Using an Outbred Murine Strain
- PMID: 29636738
- PMCID: PMC5881104
- DOI: 10.3389/fmicb.2018.00565
A Versatile New Model of Chemically Induced Chronic Colitis Using an Outbred Murine Strain
Abstract
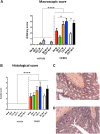
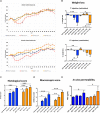
Murine colitis models are crucial tools for understanding intestinal homeostasis and inflammation. However, most current models utilize a highly inbred strain of mice, and often only one sex is employed to limit bias. This targeted approach, which in itself is biased, means that murine genetic diversity and sex-related differences are ignored, making it even more difficult to extend findings to humans, who are highly heterogeneous. Furthermore, most models do not examine the chronic form of colitis, an important fact taking into account the chronic nature of the inflammatory bowel diseases (IBD). Here, we attempted to create a more realistic murine colitis model by addressing these three issues. Using chemically induced chronic colon inflammation in an outbred strain of mice (RjOrl:SWISS [CD-1]), we (i) mimicked the relapsing nature of the disease, (ii) better represented normal genetic variability, and (iii) employed both female and male mice. Colitis was induced by intrarectal administration of dinitrobenzene sulfonic acid (DNBS). After a recovery period and 3 days before the mice were euthanized, colitis was reactivated by a second administration of DNBS. Protocol length was 24 days. Colitis severity was assessed using body mass, macroscopic scores, and histological scores. Myeloperoxidase (MPO) activity, cytokine levels, and lymphocyte populations were also characterized. Our results show that the intrarectal administration of DNBS effectively causes colitis in both female and male CD-1 mice in a dose-dependent manner, as reflected by loss of body mass, macroscopic scores and histological scores. Furthermore, colon cytokine levels and mesenteric lymph node characteristics indicate that this model involves immune system activation. Although some variables were sex-specific, most of the results support including both females and males in the model. Our ultimate goal is to make this model available to researchers for testing candidate anti-inflammatory agents, such as classical or next-generation probiotics; we also aim for the results to be more easily transferrable to human trials.
Keywords: CD-1 mice; DNBS; colitis; gut inflammation; murine IBD model.
Figures





References
-
- Elson C. O., Cong Y., McCracken V. J., Dimmitt R. A., Lorenz R. G., Weaver C. T. (2005). Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 206 260–276. 10.1111/j.0105-2896.2005.00291.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

