COX-2 contributes to LPS-induced Stat3 activation and IL-6 production in microglial cells
- PMID: 29636886
- PMCID: PMC5883137
COX-2 contributes to LPS-induced Stat3 activation and IL-6 production in microglial cells
Abstract
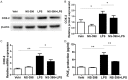
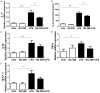
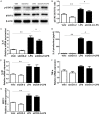
Many stimuli including lipopolysaccharide (LPS) could activate microglial cells to subsequently cause inflammatory nerve injury. However, the mechanism of LPS-induced neuroinflammation in microglial cells is still elusive. Thus, the present study was undertaken to examine the role of COX-2 in mediating the activation of Stat3 and the production of IL-6 in BV2 cells challenged with LPS. After 24 h treatment, LPS dose-dependently enhanced COX-2 expression at both mRNA and protein levels. Meanwhile, IL-6 with other inflammatory cytokines including IL-1β, TNF-α, and MCP-1 were similarly enhanced by LPS. Then a specific COX-2 inhibitor (NS-398) was administered to BV2 before LPS treatment. Significantly, COX-2 inhibition suppressed the upregulation of IL-6 at both mRNA and protein levels in line with the trend blockade on IL-1β, TNF-α, and MCP-1. Stat3 drives proinflammatory signaling pathways and contributes to IL-6 production via a transcriptional mechanism in many diseases. Here we found that inhibition of COX-2 entirely blocked LPS-induced Stat3 phosphorylation, which might contribute to the blockade of IL-6 production to some extent. Meanwhile, COX-2 siRNA approach largely reproduced the phenotypes shown by specific COX-2 inhibitor in LPS-treated BV2 cells. Together, these findings suggested that COX-2 might contribute to LPS-induced IL-6 production possibly through activating Stat3 signaling pathway in microglial cells.
Keywords: COX-2; IL-6; LPS; Microglial cells; Stat3.
Conflict of interest statement
None.
Figures




References
-
- Weng L, Zhang H, Li X, Zhan H, Chen F, Han L, Xu Y, Cao X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-kappaB and JAK2/STAT3 signaling pathways in microglia. Int Immunopharmacol. 2017;44:1–8. - PubMed
-
- Xia CY, Zhang S, Gao Y, Wang ZZ, Chen NH. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharmacol. 2015;25:377–382. - PubMed
-
- Okorji UP, Velagapudi R, El-Bakoush A, Fiebich BL, Olajide OA. Antimalarial drug artemether inhibits neuroinflammation in BV2 microglia through Nrf2-dependent mechanisms. Mol Neurobiol. 2016;53:6426–6443. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
