Extracorporeal shock wave markedly alleviates radiation-induced chronic cystitis in rat
- PMID: 29636892
- PMCID: PMC5883143
Extracorporeal shock wave markedly alleviates radiation-induced chronic cystitis in rat
Abstract
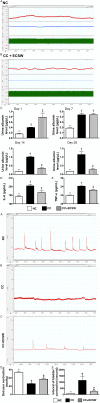
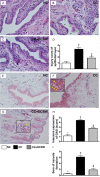
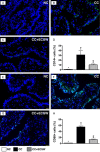
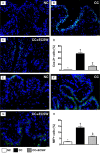
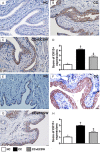
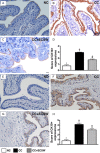
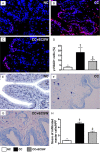
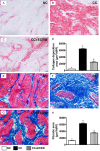
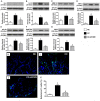
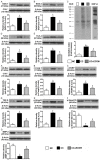
This study tested the hypothesis that extracorporeal shock wave (ECSW) treatment can effectively inhibit radiation-induced chronic cystitis (CC). Adult male Sprague-Dawley (SD) rats (n = 24) were randomly divided into group 1 (normal control), group 2 (CC induced by radiation with 300 cGy twice with a four-hour interval to the urinary bladder), group 3 [CC with ECSW treatment (0.2 mJ/mm2/120 impulses/at days 1, 7, and 14 after radiation)]. Bladder specimens were harvested by day 28 after radiation. By day 28 after radiation, the degree of detrusor contraction impairment was significantly higher in group 2 than that in groups 1 and 3, and significantly higher in group 3 than that in group 1 (P<0.0001). The urine albumin concentration expressed an opposite pattern compared to that of detrusor function among the three groups (P<0.0001). The bladder protein expressions of inflammatory (TLR-2/TLR-4/IL-6/IL-12/MMP-9/TNF-α/NF-κB/RANTES/iNOS) and oxidative-stress (NOX-1/NOX-2/oxidized protein) biomarkers exhibited a pattern identical to that of urine albumin in all groups (all P<0.0001). The cellular expressions of inflammatory (CD14+/CD68+/CD74+/COX-2/MIF+/substance P+) and cytokeratin (CK13+/HMW CK+/CK+17/CK+18/CK+19) biomarkers, and collagen-deposition/fibrotic areas as well as epithelial-damaged score displayed an identical pattern compared to that of urine albumin among the three groups (all P<0.0001). In conclusion, ECSW treatment effectively protected urinary bladder from radiation-induced CC.
Keywords: Chronic cystitis; extracorporeal shock wave; inflammation; oxidative stress; radiotherapy.
Conflict of interest statement
None.
Figures
References
-
- Stewart B, Wild C. World cancer report. Lyon CEDEX, France: 2014. Chap 5.12. 465-481.
-
- Alesawi AM, El-Hakim A, Zorn KC, Saad F. Radiation-induced hemorrhagic cystitis. Curr Opin Support Palliat Care. 2014;8:235–240. - PubMed
-
- Grellety T, Houede N, Hoepffner JL, Riviere J, Merino C, Lieutenant V, Gross-Goupil M, Richaud P, Dupin C, Sargos P, Roubaud G. Hemorrhagic cystitis in patients treated with cabazitaxel: a radiation recall syndrome? Ann Oncol. 2014;25:1248–1249. - PubMed
-
- Mendenhall WM, Henderson RH, Costa JA, Hoppe BS, Dagan R, Bryant CM, Nichols RC, Williams CR, Harris SE, Mendenhall NP. Hemorrhagic radiation cystitis. Am J Clin Oncol. 2015;38:331–336. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
