Anticancer efficacies of arsenic disulfide through apoptosis induction, cell cycle arrest, and pro-survival signal inhibition in human breast cancer cells
- PMID: 29636995
- PMCID: PMC5883090
Anticancer efficacies of arsenic disulfide through apoptosis induction, cell cycle arrest, and pro-survival signal inhibition in human breast cancer cells
Abstract
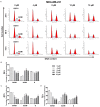
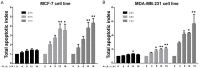
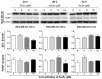
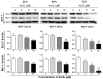
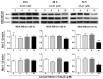
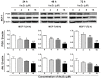
Arsenic disulfide, a major effective component of realgar, has been investigated for its anti-cancer potential and shown to have therapeutic efficacies in hematological and some solid tumors. However, its effect against breast cancer is rarely reported. In this study, we investigated the anti-cancer effects of As2S2 in human breast cancer cell lines MCF-7 and MDA-MB-231, and further elucidated its underlying mechanisms. As2S2 significantly inhibited cell viabilities, induced apoptosis, and led to cell cycle arrest in both cell lines with a dose- and time-dependent manner. As2S2 upregulated pro-apoptotic proteins like p53 and PARP in MCF-7 cells. Besides, As2S2 downregulated anti-apoptotic proteins like Bcl-2 and Mcl-1, as well as cell cycle-related proteins cyclin A2 and cyclin D1 in both cell lines. Of note, the expression level of cyclin B1 was downregulated in MCF-7 cells, whereas, upregulated in MDA-MB-231 cells. Moreover, As2S2 significantly inhibited the pro-survival signals in PI3K/Akt pathway in both cell lines. In conclusion, As2S2 inhibited cell viabilities, induced apoptosis and cell cycle arrest in both MCF-7 and MDA-MB-231 cell lines by regulating the expression of key proteins involved in related pathways. These results provide fundamental insights into the clinical application of As2S2 for treatment of patients with breast cancer.
Keywords: Arsenic disulfide; MCF-7; MDA-MB-231; apoptosis; cell cycle; cell viability.
Conflict of interest statement
None.
Figures







References
-
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26:1275–1281. - PubMed
-
- Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C, Högel B, Darb-Esfahani S, Mehta K, Loibl S. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124:133–140. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
