Development of a core outcome set for clinical trials in childhood constipation: a study using a Delphi technique
- PMID: 29637094
- PMCID: PMC5842998
- DOI: 10.1136/bmjpo-2017-000017
Development of a core outcome set for clinical trials in childhood constipation: a study using a Delphi technique
Abstract
Objective: Patients, their parents and healthcare professionals (HCPs) have a different perception regarding the symptoms of functional constipation (FC). Consequently, a lack of agreement exists on definitions and outcomes used in therapeutic trials of FC. Therefore, our aim was to develop a core outcome set (COS) for FC for children aged 0-1 year and 1-18 years.
Design and setting: Prospective study design: primary, secondary and tertiary care settings.
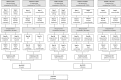
Methods: This COS was developed using a Delphi technique. First, HCPs, parents of children with FC and patients aged ≥12-18 years were asked to list up to five outcomes they considered relevant in the treatment of FC. Outcomes mentioned by >10% of participants were included in a shortlist. In the next phase, outcomes on this shortlist were rated and prioritised by HCPs, parents and patients. Outcomes with the highest scores were included in a draft COS. In a face-to-face expert meeting, the final COS was determined.
Results: The first phase was completed by 109 HCPs, 165 parents and 50 children. Fifty HCPs, 80 parents and 50 children completed the subsequent phase. The response rate was between 63% and 100% in both steps. The final COS for all ages consisted of: defecation frequency, stool consistency, painful defecation, quality of life, side effects of treatment, faecal incontinence, abdominal pain and school attendance.
Conclusion: The use of this COS for FC will decrease study heterogeneity and improve comparability of studies. Therefore, researchers are recommended to use this COS in future therapeutic trials on childhood FC.
Keywords: functional constipation; outcomes measures; paediatrics; parent reported outcomes; patient reported outcomes.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011;25:3–18. doi:10.1016/j.bpg.2010.12.010 - DOI - PubMed
-
- Kuizenga-Wessel S, Benninga MA, Tabbers MM. Reporting outcome measures of functional constipation in children from 0 to 4 years of age. J Pediatr Gastroenterol Nutr 2015;60:446–56. doi:10.1097/MPG.0000000000000631 - DOI - PubMed
-
- Baker SS, Liptak GS, Colletti RB, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr 1999;29:612–26. - PubMed
-
- Boccia G, Manguso F, Coccorullo P, et al. Functional defecation disorders in children: PACCT criteria versus Rome II criteria. J Pediatr 2007;151:e1:394–98. doi:10.1016/j.jpeds.2007.04.011 - DOI - PubMed
-
- Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2006;130:1527–37. doi:10.1053/j.gastro.2005.08.063 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources