Validation of a classification system for treatment-related mortality in children with cancer
- PMID: 29637120
- PMCID: PMC5862234
- DOI: 10.1136/bmjpo-2017-000082
Validation of a classification system for treatment-related mortality in children with cancer
Abstract
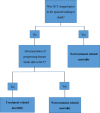
Background: Death not directly due to cancer has been termed 'treatment-related mortality' (TRM). Appreciating the differences between TRM and disease-related death is critical in directing strategies to improve supportive care, interventions delivered or disease progression. Recently, a global collaboration developed and validated a consensus-based classification tool and attribution system.
Objectives: To evaluate the reliability of the newly developed consensus-based definition of TRM and explore the use of the cause-of-death attribution system outside the centre it was initially validated (Toronto, Canada). In the initial study, reviewers listed multiple causes of death. In this study, reviewers identified a primary cause for simplicity.
Setting: The paediatric haematology and oncology department at Leeds Teaching Hospital in Leeds, UK.
Participants: Two consultants and two clinical research associates (CRAs).
Methods: Thirty medical records of the most recent deaths in children with cancer, 2 and 4 weeks prior to death, were anonymised and presented to the participants. Reviewers independently classified deaths as 'treatment related mortality' or 'not treatment related' according to the algorithm developed. When TRM occurred, reviewers applied the cause-of-death attribution system to identify the primary cause of death. Inter-relater reliability was assessed using the kappa statistic (k).
Main outcome: Inter-relater reliability between CRA and consultants.
Results: Reliability of the classification was deemed 'very good' between CRA and consultants (k=0.86, 95% CI 0.72 to 0.97). Ten deaths were classified as TRM, of which infection was the most frequent cause identified. Reviewers disagreed on the primary cause of death (eg, respiratory vs infection) when applying the cause-of-death attribution system in six cases and probable and possible causes in four cases. The study identified how the algorithm may not detect TRM in patients receiving non-curative therapy.
Conclusions: The classification and cause of death attribution system could be implemented in different healthcare settings. Adaptation of the classification tool in patients receiving non-curative interventions and the cause of death attribution system should be considered.
Keywords: haematology; oncology; palliative care.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Alexander S, Pole JD, Gibson P, et al. . Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol 2015;16:e604–e610. doi:10.1016/S1470-2045(15)00197-7 - DOI - PubMed
-
- CRUK. Children’s cancers mortality in Europe and worldwide, 2014. http://www.cancerresearchuk.org/health-professional/cancer-statistics/ch... (cited 2016 September).
-
- Kremer LC, Mulder RL, Oeffinger KC, et al. . A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 2013;60:543–9. doi:10.1002/pbc.24445 - DOI - PMC - PubMed
-
- Blanco E, Beyene J, Maloney AM, et al. . Non-relapse mortality in pediatric acute lymphoblastic leukemia: a systematic review and meta-analysis. Leuk Lymphoma 2012;53:878–85. doi:10.3109/10428194.2011.639018 - DOI - PubMed
-
- White Y, Castle VP, Haig A. Pediatric oncology in developing countries: challenges and solutions. J Pediatr 2013;162:1090–1. doi:10.1016/j.jpeds.2013.02.035 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources