Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues
- PMID: 29637607
- PMCID: PMC6039247
- DOI: 10.1002/arch.21464
Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues
Abstract
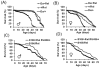
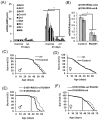
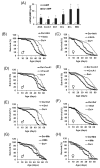
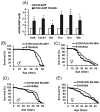
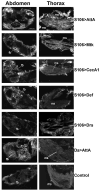
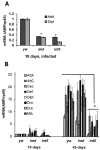
The innate immune response tends to become hyperactive and proinflammatory in older organisms. We investigated connections between activity of the immune-related genes and aging using the Drosophila model. A hallmark of Drosophila immunity is the production of antimicrobial peptides (AMP), whose expression is triggered via activation of the Toll and Imd immune pathways and regulated by NF-ĸB-like transcription factors, Dif/Dorsal and Relish. It was previously shown that overexpression of the upstream component of the immune pathways shortens lifespan via activation of the Relish-dependent immune response. Here we show that direct overexpression of the Relish target AMP genes broadly at high levels or in the fat body induced apoptosis, elicited depolarization of the mitochondria and significantly shortened lifespan. Underexpression of Relish in the fat body beginning in the second half of lifespan prevented overactivation of AMPs and extended longevity. Unlike infection-induced responses, the age-related increase in AMPs does not require the upstream recognition/transduction module of the Imd pathway. It does however require downstream elements, including Relish and Ird5, a component of the downstream IKK complex. Together, these results established causal links between high-level production of antimicrobial peptides and longevity.
Keywords: Drosophila; aging; antimicrobial peptide; cytotoxicity; immunity.
© 2018 Wiley Periodicals, Inc.
Figures



References
-
- Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 2012;12:153–65. - PubMed
-
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999;23:329–44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

