Melatonin Treatment Enhances Aβ Lymphatic Clearance in a Transgenic Mouse Model of Amyloidosis
- PMID: 29637859
- PMCID: PMC8803126
- DOI: 10.2174/1567205015666180411092551
Melatonin Treatment Enhances Aβ Lymphatic Clearance in a Transgenic Mouse Model of Amyloidosis
Abstract
Background: It has been postulated that inadequate clearance of the amyloid β protein (Aβ) plays an important role in the accumulation of Aβ in sporadic late onset Alzheimer's disease (AD). While the blood brain barrier (BBB) has taken the center stage in processes involving Aβ clearance, little information is available about the role of the lymphatic system. We previously reported that Aβ is cleared through the lymphatic system. We now assessed lymphatic Aβ clearance by treating a mouse model of AD amyloidosis with melatonin, an Aβ aggregation inhibitor and immuno-regulatory neurohormone.
Objective: To confirm and expand our initial finding that Aβ is cleared through the lymphatic system. Lymphatic clearance of metabolic and cellular "waste" products from the brain into the peripheral lymphatic system has been known for a long time. However, except for our prior report, there is no additional experimental data published about Aβ being cleared into peripheral lymph nodes.
Methods: For these experiments, we used a transgenic mouse model (Tg2576) that over-expresses a mutant form of the Aβ precursor protein (APP) in the brain. We examined levels of Aβ in plasma and in lymph nodes of transgenic mice as surrogate markers of vascular and lymphatic clearance, respectively. Aβ levels were also measured in the brain and in multiple tissues.
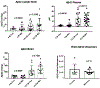
Results: Clearance of Aβ peptides through the lymphatic system was confirmed in this study. Treatment with melatonin led to the following changes: 1-A statistically significant increase in soluble monomeric Aβ40 and an increasing trend in Aβ42 in cervical and axillary lymph nodes of treated mice. 2- Statistically significant decreases in oligomeric Aβ40 and a decreasing trend Aβ42 in the brain.
Conclusion: The data expands on our prior report that the lymphatic system participates in Aβ clearance from the brain. We propose that abnormalities in Aβ clearance through the lymphatic system may contribute to the development of cerebral amyloidosis. Melatonin and related indole molecules (i.e., indole- 3-propionic acid) are known to inhibit Aβ aggregation although they do not reverse aggregated Aβ or amyloid fibrils. Therefore, these substances should be further explored in prevention trials for delaying the onset of cognitive impairment in high risk populations.
Keywords: Alzheimer's disease; amyloid clearance; beta amyloid; lymphatic nodes; melatonin; transgenic mice..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures

References
-
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122(3):1131–5. - PubMed
-
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, et al. A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia. Ann Neurol. 2008;63(3):377–87. - PubMed
-
- Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer's disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. The American journal of pathology. 1998;152(4):871–7. - PMC - PubMed
-
- Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998;70(5):2212–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

