Development of a Validated Interferon Score Using NanoString Technology
- PMID: 29638206
- PMCID: PMC5963606
- DOI: 10.1089/jir.2017.0127
Development of a Validated Interferon Score Using NanoString Technology
Abstract
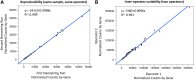
Chronic elevation of interferon (IFN)-response genes (IRG) in a subset of patients with systemic immune-dysregulatory diseases, including the Mendelian Type-I IFN-mediated autoinflammatory diseases and some autoimmune diseases suggest a causative role of excessive IFN signaling in the disease pathogenesis and as target for treatment. We developed a 28-IFN response gene scoring system to calculate either a standardized or geomean score by customizing a NanoString assay to quantify the expression of putative IRGs. The gene targets were selected in patients with the IFN-mediated disease chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) and an adult patient with chronic hepatitis C who received the first dose of pegylated interferon alpha-2a. The putative target genes were validated in patients with STING-associated vasculopathy with onset in infancy (SAVI), a monogenic autoinflammatory disease caused by gain-of-function mutations in TMEM173 that encodes the viral sensor stimulator of IFN genes (STING), and had low expression in clinically active patients with the monogenic IL-1-mediated autoinflammatory disease, neonatal-onset multisystem inflammatory disease (NOMID) and in healthy controls. The score calculation on the NanoString assay is rapid and showed high reproducibility and low intra-, and interassay variability. The utility of this 28-gene IFN score may be explored in the diagnosis of patients with presumed interferonopathies and as a biomarker to assess disease activity, long-term outcome, and treatment responses.
Trial registration: ClinicalTrials.gov NCT02974595 NCT00718172.
Keywords: IFN score; IFN-mediated autoinflammatory diseases; NanoString assay; assay validation; blood IFN signature; interferonopathies.
Conflict of interest statement
The authors have no relevant disclosures. R.G.M. has received grant support from SOBI, Novartis, Regeneron, and Eli Lilly for clinical studies.
Figures

References
-
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. 2007. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med 13(5):543–551 - PubMed
-
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100(5):2610–2615 - PMC - PubMed
-
- Brehm A, Liu Y, Sheikh A, Marrero B, Omoyinmi E, Zhou Q, Montealegre G, Biancotto A, Reinhardt A, Almeida de Jesus A, Pelletier M, Tsai WL, Remmers EF, Kardava L, Hill S, Kim H, Lachmann HJ, Megarbane A, Chae JJ, Brady J, Castillo RD, Brown D, Casano AV, Gao L, Chapelle D, Huang Y, Stone D, Chen Y, Sotzny F, Lee CC, Kastner DL, Torrelo A, Zlotogorski A, Moir S, Gadina M, McCoy P, Wesley R, Rother K, Hildebrand PW, Brogan P, Kruger E, Aksentijevich I, Goldbach-Mansky R. 2015. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest 125(11):4196–4211 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

