Memory deficiency, cerebral amyloid angiopathy, and amyloid-β plaques in APP+PS1 double transgenic rat model of Alzheimer's disease
- PMID: 29641600
- PMCID: PMC5895023
- DOI: 10.1371/journal.pone.0195469
Memory deficiency, cerebral amyloid angiopathy, and amyloid-β plaques in APP+PS1 double transgenic rat model of Alzheimer's disease
Abstract
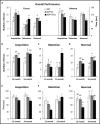
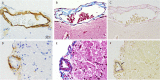
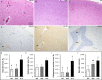
Transgenic rat models of Alzheimer's disease were used to examine differences in memory and brain histology. Double transgenic female rats (APP+PS1) over-expressing human amyloid precursor protein (APP) and presenilin 1 (PS1) and single transgenic rats (APP21) over-expressing human APP were compared with wild type Fischer rats (WT). The Barnes maze assessed learning and memory and showed that both APP21 and APP+PS1 rats made significantly more errors than the WT rats during the acquisition phase, signifying slower learning. Additionally, the APP+PS1 rats made significantly more errors following a retention interval, indicating impaired memory compared to both the APP21 and WT rats. Immunohistochemistry using an antibody against amyloid-β (Aβ) showed extensive and mostly diffuse Aβ plaques in the hippocampus and dense plaques that contained tau in the cortex of the brains of the APP+PS1 rats. Furthermore, the APP+PS1 rats also showed vascular changes, including cerebral amyloid angiopathy with extensive Aβ deposits in cortical and leptomeningeal blood vessel walls and venous collagenosis. In addition to the Aβ accumulation observed in arterial, venous, and capillary walls, APP+PS1 rats also displayed enlarged blood vessels and perivascular space. Overall, the brain histopathology and behavioral assessment showed that the APP+PS1 rats demonstrated behavioral characteristics and vascular changes similar to those commonly observed in patients with Alzheimer's disease.
Conflict of interest statement
Figures



References
-
- Alzheimer’s Association. 2017 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2017;13:325–73.
-
- Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004;23:105–14. doi: 10.1385/JMN:23:1-2:105 - DOI - PubMed
-
- Attems J. Sporadic cerebral amyloid angiopathy: pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol. 2005;110:345–59. doi: 10.1007/s00401-005-1074-9 - DOI - PubMed
-
- Weller RO, Boche D, Nicoll JA. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z - DOI - PubMed
-
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

