A Universal Approach to Optimize the Folding and Stability of Prefusion-Closed HIV-1 Envelope Trimers
- PMID: 29642014
- PMCID: PMC6010203
- DOI: 10.1016/j.celrep.2018.03.061
A Universal Approach to Optimize the Folding and Stability of Prefusion-Closed HIV-1 Envelope Trimers
Abstract
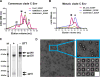
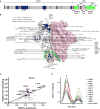
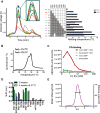
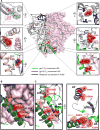
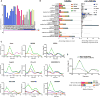
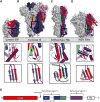
The heavily glycosylated native-like envelope (Env) trimer of HIV-1 is expected to have low immunogenicity, whereas misfolded forms are often highly immunogenic. High-quality correctly folded Envs may therefore be critical for developing a vaccine that induces broadly neutralizing antibodies. Moreover, the high variability of Env may require immunizations with multiple Envs. Here, we report a universal strategy that provides for correctly folded Env trimers of high quality and yield through a repair-and-stabilize approach. In the repair stage, we utilized a consensus strategy that substituted rare strain-specific residues with more prevalent ones. The stabilization stage involved structure-based design and experimental assessment confirmed by crystallographic feedback. Regions important for the refolding of Env were targeted for stabilization. Notably, the α9-helix and an intersubunit β sheet proved to be critical for trimer stability. Our approach provides a means to produce prefusion-closed Env trimers from diverse HIV-1 strains, a substantial advance for vaccine development.
Keywords: ConC_base; HIV; SOSIP; X-ray structure; chronic; envelope protein; hybrid sheet; stabilization; transmitted/founder; vaccine.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
These studies were funded by Janssen Vaccines and Prevention. L.R., D.T., D.v.M., S.B., N.M.S., A.K., I.J.M.B., H.S., and J.P.M.L. are employees at Janssen. L.R., D.T., N.M.S., A.K., and J.P.M.L. are inventors on an international patent application describing trimer stabilizing HIV envelope protein mutations. The remaining authors declare no competing interests.
Figures
References
-
- Binley JM, Sanders RW, Clas B, Schuelke N, Master A, Guo Y, Kajumo F, Anselma DJ, Maddon PJ, Olson WC, Moore JP. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

