Genome-wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication
- PMID: 29642015
- PMCID: PMC5939577
- DOI: 10.1016/j.celrep.2018.03.045
Genome-wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication
Abstract
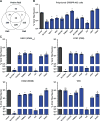
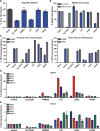
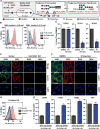
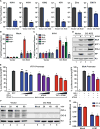
The emergence of influenza A viruses (IAVs) from zoonotic reservoirs poses a great threat to human health. As seasonal vaccines are ineffective against zoonotic strains, and newly transmitted viruses can quickly acquire drug resistance, there remains a need for host-directed therapeutics against IAVs. Here, we performed a genome-scale CRISPR/Cas9 knockout screen in human lung epithelial cells with a human isolate of an avian H5N1 strain. Several genes involved in sialic acid biosynthesis and related glycosylation pathways were highly enriched post-H5N1 selection, including SLC35A1, a sialic acid transporter essential for IAV receptor expression and thus viral entry. Importantly, we have identified capicua (CIC) as a negative regulator of cell-intrinsic immunity, as loss of CIC resulted in heightened antiviral responses and restricted replication of multiple viruses. Therefore, our study demonstrates that the CRISPR/Cas9 system can be utilized for the discovery of host factors critical for the replication of intracellular pathogens.
Keywords: CIC; CRISPR/Cas9 screen; Capicua; GeCKO; H5N1; SLC35A1; cell-intrinsic immunity; host factors; influenza virus; sialic acid pathway.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

