Oral Vaccination with a DNA Vaccine Encoding Capsid Protein of Duck Tembusu Virus Induces Protection Immunity
- PMID: 29642401
- PMCID: PMC5923474
- DOI: 10.3390/v10040180
Oral Vaccination with a DNA Vaccine Encoding Capsid Protein of Duck Tembusu Virus Induces Protection Immunity
Abstract
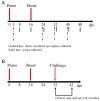
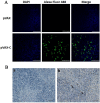
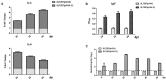
The emergence of duck tembusu virus (DTMUV), a new member of the Flavivirus genus, has caused great economical loss in the poultry industry in China. Since the outbreak and spread of DTMUV is hard to control in a clinical setting, an efficient and low-cost oral delivery DNA vaccine SL7207 (pVAX1-C) based on the capsid protein of DTMUV was developed and evaluated in this study. The antigen capsid protein was expressed from the DNA vaccine SL7207 (pVAX1-C), both in vitro and in vivo. The humoral and cellular immune responses in vivo were observed after oral immunization with the SL7207 (pVAX1-C) DNA vaccine. High titers of the specific antibody against the capsid protein and the neutralizing antibody against the DTMUV virus were both detected after inoculation. The ducks were efficiently protected from lethal DTMUV exposure by the SL7207 (pVAX1-C) vaccine in this experiment. Taken together, we demonstrated that the capsid protein of DTMUV possesses a strong immunogenicity against the DTMUV infection. Moreover, an oral delivery of the DNA vaccine SL7207 (pVAX1-C) utilizing Salmonella SL7207 was an efficient way to protect the ducks against DTMUV infection and provides an economic and fast vaccine delivery strategy for a large scale clinical use.
Keywords: Flavivirus; capsid protein; duck tembusu virus; immunogenicity; oral DNA vaccine.
Conflict of interest statement
The author declare that they have no competing interests.
Figures

References
-
- Thai K.T., Nga T.T., van Nam N., Phuong H.L., Giao P.T., Hung le Q., Binh T.Q., van Doornum G.J., de Vries P.J. Incidence of primary dengue virus infections in southern vietnamese children and reactivity against other flaviviruses. Trop. Med. Int. Health. 2007;12:1553–1557. doi: 10.1111/j.1365-3156.2007.01964.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

