A Single Dose of Atorvastatin Applied Acutely after Spinal Cord Injury Suppresses Inflammation, Apoptosis, and Promotes Axon Outgrowth, Which Might Be Essential for Favorable Functional Outcome
- PMID: 29642434
- PMCID: PMC5979414
- DOI: 10.3390/ijms19041106
A Single Dose of Atorvastatin Applied Acutely after Spinal Cord Injury Suppresses Inflammation, Apoptosis, and Promotes Axon Outgrowth, Which Might Be Essential for Favorable Functional Outcome
Abstract
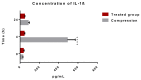
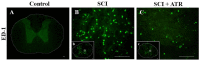
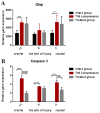
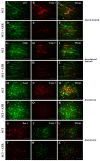
The aim of our study was to limit the inflammatory response after a spinal cord injury (SCI) using Atorvastatin (ATR), a potent inhibitor of cholesterol biosynthesis. Adult Wistar rats were divided into five experimental groups: one control group, two Th9 compression (40 g/15 min) groups, and two Th9 compression + ATR (5 mg/kg, i.p.) groups. The animals survived one day and six weeks. ATR applied in a single dose immediately post-SCI strongly reduced IL-1β release at 4 and 24 h and considerably reduced the activation of resident cells at one day post-injury. Acute ATR treatment effectively prevented the excessive infiltration of destructive M1 macrophages cranially, at the lesion site, and caudally (by 66%, 62%, and 52%, respectively) one day post-injury, whereas the infiltration of beneficial M2 macrophages was less affected (by 27%, 41%, and 16%). In addition, at the same time point, ATR visibly decreased caspase-3 cleavage in neurons, astrocytes, and oligodendrocytes. Six weeks post-SCI, ATR increased the expression of neurofilaments in the dorsolateral columns and Gap43-positive fibers in the lateral columns around the epicenter, and from day 30 to 42, significantly improved the motor activity of the hindlimbs. We suggest that early modulation of the inflammatory response via effects on the M1/M2 macrophages and the inhibition of caspase-3 expression could be crucial for the functional outcome.
Keywords: Atorvastatin; Gap43; caspase-3; gene expression; inflammatory response; macrophages; neurofilaments; spinal cord compression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


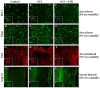
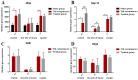
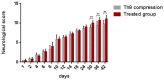
References
-
- Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011;71:281–299. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

