MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing
- PMID: 29642447
- PMCID: PMC5951453
- DOI: 10.3390/ma11040569
MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing
Abstract
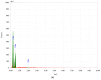
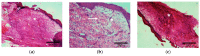
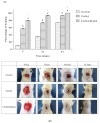
Two self-associating biopolymers, namely chitosan (Ch) and a high-molar-mass hyaluronan (HA), were used to prepare membranes with the aim to protect and to enhance the healing of injured skin. A mitochondrially-targeted antioxidant-MitoQ-was incorporated into the mixture of biopolymers prior to their self-association. These three-component membranes were evaluated in detail utilising surface roughness measurements, contact angle measurements, hemocompatibility, and thrombogenicity analyses. Furthermore, in vivo application of Ch/HA/MitoQ membranes was assessed on injured rabbit and rat skin utilizing histological methods. The results showed that the prepared thrombogenic Ch/HA/MitoQ membranes had higher roughness, which allowed for greater surface area for tissue membrane interaction during the healing processes, and lower cytotoxicity levels than controls. MitoQ-loaded composite membranes displayed superior healing properties in these animal models compared to control membranes.
Keywords: chitosan; hyaluronan; mitochondrially-targeted antioxidant; skin wounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

