In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity
- PMID: 29642509
- PMCID: PMC5923559
- DOI: 10.3390/nano8040229
In-Situ Synthesis of Hydrogen Titanate Nanotube/Graphene Composites with a Chemically Bonded Interface and Enhanced Visible Photocatalytic Activity
Abstract
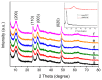
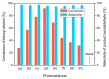
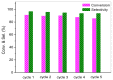
Hydrogen titanate nanotube (HTT)/graphene nanocomposites are synthesized by hydrothermal reduction of graphene oxide (GO) and simultaneous preparation of nanotubular HTT via an alkaline hydrothermal process. By using this facile in-situ compositing strategy, HTT are densely supported upon the surface of graphene sheets with close interface contacts. The as-prepared HTT/graphene nanocomposites possess significantly enhanced visible light catalytic activity for the partial oxidation of benzylic alcohols. The amount of graphene has significant influence on catalytic activity and the optimal content of graphene is 1.0 wt %, giving a normalized rate constant k of 1.71 × 10-3 g/m²·h, which exceeds that of pure HTT and HTT/graphene-1.0% mixed by a factor of 7.1 or 5.2. Other than the general role of graphene as a high-performance electron acceptor or transporter, the observed enhancement in photocatalytic activity over HTT/graphene can be ascribed to the improved interfacial charge migration from enhanced chemical bonding (Ti-C bonds) during the in-situ compositing process. The formation of Ti-C bonds is confirmed by XPS analysis and the resulting enhanced separation of photoinduced charge carriers is demonstrated by electrochemical impedance spectra and transient photocurrent response.
Keywords: Ti–C bonds; benzylic alcohols; hydrogen titanate; interfacial charge transfer; photocatalytic selective oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Bismuth oxyiodide-graphene nanocomposites with high visible light photocatalytic activity.J Colloid Interface Sci. 2013 May 15;398:161-7. doi: 10.1016/j.jcis.2013.02.007. Epub 2013 Feb 24. J Colloid Interface Sci. 2013. PMID: 23499294
-
Enhanced photocatalytic H₂-production activity of graphene-modified titania nanosheets.Nanoscale. 2011 Sep 1;3(9):3670-8. doi: 10.1039/c1nr10610d. Epub 2011 Aug 8. Nanoscale. 2011. PMID: 21826308
-
Hydrothermal synthesis of CaIn2S4-reduced graphene oxide nanocomposites with increased photocatalytic performance.ACS Appl Mater Interfaces. 2014 Aug 13;6(15):12877-84. doi: 10.1021/am5028296. Epub 2014 Jul 14. ACS Appl Mater Interfaces. 2014. PMID: 24998484
-
Unique photocatalytic oxidation reactivity and selectivity of TiO₂-graphene nanocomposites.Nanoscale. 2012 May 21;4(10):3193-200. doi: 10.1039/c2nr30427a. Epub 2012 Apr 5. Nanoscale. 2012. PMID: 22481610
-
Synthesis of GO supported Fe2O3-TiO2 nanocomposites for enhanced visible-light photocatalytic applications.Dalton Trans. 2015 Sep 28;44(36):16024-35. doi: 10.1039/c5dt02983j. Dalton Trans. 2015. PMID: 26286295
Cited by
-
Facile Synthesis of Novel CaIn₂S₄/ZnIn₂S₄ Composites with Efficient Performance for Photocatalytic Reduction of Cr(VI) under Simulated Sunlight Irradiation.Nanomaterials (Basel). 2018 Jun 27;8(7):472. doi: 10.3390/nano8070472. Nanomaterials (Basel). 2018. PMID: 29954127 Free PMC article.
References
-
- Kamat P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C. 2007;111:2834–2860. doi: 10.1021/jp066952u. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

