Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections
- PMID: 29642603
- PMCID: PMC6027529
- DOI: 10.3390/pharmaceutics10020046
Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections
Abstract
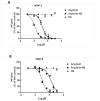
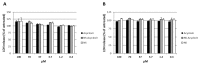
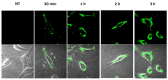
Acyclovir is not a good candidate for passive permeation since its polarity and solubility limit is partitioning into the stratum corneum. This work aims to develop a new topical formulation for the acyclovir delivery. New chitosan nanospheres (NS) were prepared by a modified nano-emulsion template method. Chitosan NS were characterized by Dynamic Light Scattering (DLS), Transmission Electron Microscopy (TEM), and an in vitro release study. The in vitro skin permeation experiment was carried out using Franz cells and was equipped with porcine skin. Biological studies were performed on the Vero cell line infected by HSV-1 and HSV-2 strains. The acyclovir loaded chitosan NS appeared with a spherical shape, a size of about 200 nm, and a negative zeta potential of about 40.0 mV. The loading capacity of the drug was about 8.5%. In vitro release demonstrated that the percentage of acyclovir delivered from the nanospheres was approximately 30% after six hours. The in vitro skin permeation studies confirmed an improved amount of permeated acyclovir. The acyclovir-NS complex displayed a higher antiviral activity than that of free acyclovir against both the HSV-1 and the HSV-2 strain. The acyclovir-loaded NS showed no anti-proliferative activity and no signs of cytotoxicity induced by NS was detected. Confocal laser scanning microscopy confirmed that the NS are taken up by the cells.
Keywords: acyclovir; antiviral activity; chitosan nanospheres; topical infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Roizman B., Knipe D.M., Whitley R. Herpes Simplex Viruses. In: Knipe D.M., Howley P., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 2501–2601.
-
- World Health Organization, Media Centre—Herpes Simplex Virus. [(accessed on 28 February 2018)];2017 Available online: http://www.who.int/mediacentre/factsheets/fs400/en/
-
- Who Guidelines for the Treatment of Genital Herpes Simplex Virus. World Health Organizations, WHO Document Production Services; Geneva, Switzerland: 2016. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

