Signals of Systemic Immunity in Plants: Progress and Open Questions
- PMID: 29642641
- PMCID: PMC5979450
- DOI: 10.3390/ijms19041146
Signals of Systemic Immunity in Plants: Progress and Open Questions
Abstract
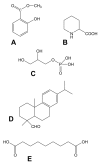
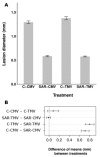
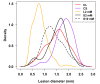
Systemic acquired resistance (SAR) is a defence mechanism that induces protection against a wide range of pathogens in distant, pathogen-free parts of plants after a primary inoculation. Multiple mobile compounds were identified as putative SAR signals or important factors for influencing movement of SAR signalling elements in Arabidopsis and tobacco. These include compounds with very different chemical structures like lipid transfer protein DIR1 (DEFECTIVE IN INDUCED RESISTANCE1), methyl salicylate (MeSA), dehydroabietinal (DA), azelaic acid (AzA), glycerol-3-phosphate dependent factor (G3P) and the lysine catabolite pipecolic acid (Pip). Genetic studies with different SAR-deficient mutants and silenced lines support the idea that some of these compounds (MeSA, DIR1 and G3P) are activated only when SAR is induced in darkness. In addition, although AzA doubled in phloem exudate of tobacco mosaic virus (TMV) infected tobacco leaves, external AzA treatment could not induce resistance neither to viral nor bacterial pathogens, independent of light conditions. Besides light intensity and timing of light exposition after primary inoculation, spectral distribution of light could also influence the SAR induction capacity. Recent data indicated that TMV and CMV (cucumber mosaic virus) infection in tobacco, like bacteria in Arabidopsis, caused massive accumulation of Pip. Treatment of tobacco leaves with Pip in the light, caused a drastic and significant local and systemic decrease in lesion size of TMV infection. Moreover, two very recent papers, added in proof, demonstrated the role of FMO1 (FLAVIN-DEPENDENT-MONOOXYGENASE1) in conversion of Pip to N-hydroxypipecolic acid (NHP). NHP systemically accumulates after microbial attack and acts as a potent inducer of plant immunity to bacterial and oomycete pathogens in Arabidopsis. These results argue for the pivotal role of Pip and NHP as an important signal compound of SAR response in different plants against different pathogens.
Keywords: Arabidopsis; N-hydroxypipecolic acid; SAR signalling; azelaic acid; glycerol-3-phosphate; light dependent signalling; methyl salicylate; pipecolic acid; salicylic acid; spectral distribution of light; tobacco.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

