Regulation of Cell Cycle Regulatory Proteins by MicroRNAs in Uterine Leiomyoma
- PMID: 29642801
- PMCID: PMC6728566
- DOI: 10.1177/1933719118768692
Regulation of Cell Cycle Regulatory Proteins by MicroRNAs in Uterine Leiomyoma
Abstract
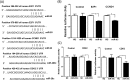
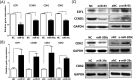
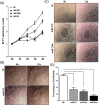
The objective of this study was to determine whether miR-93, miR-29c, and miR-200c, which we previously reported to be downregulated in leiomyomas, target cell cycle regulatory proteins that influence cell proliferation. Based on TargetScan algorithm 3 cell cycle regulatory proteins namely, E2F transcription factor 1 (E2F1), Cyclin D1 (CCND1) and CDK2 which were predicted to be targets of these miRNAs were further analyzed. In 30 hysterectomy specimens, we found the expression of E2F1 and CCND1 messenger RNA (mRNA) was increased in leiomyoma as compared to matched myometrium, with no significant changes in CDK2 mRNA levels. There was a significant increase in the abundance of all 3 proteins in leiomyoma in comparison with matched myometrium. Using luciferase reporter assay, we demonstrated E2F1 and CCND1 are targets of miR-93 and CDK2 is a target of miR-29c and miR-200c. We confirmed these findings through transfection studies in which transfection of primary leiomyoma cells with miR-93 resulted in a significant decrease in the expression of E2F1 and CCND1 mRNA and protein levels, whereas knockdown of miR-93 had the opposite effect. Similarly, overexpression of miR-29c and miR-200c in leiomyoma cells inhibited the expression of CDK2 protein and mRNA, whereas knockdown of this microRNAs (miRNA) had the opposite effect. Transfection of miR-29c, miR-200c, and miR-93 in primary leiomyoma cells resulted in a time-dependent inhibition of cell proliferation and cell motility. These results collectively indicate that the 3 miRNAs known to be downregulated in fibroid tumors are critical in regulation of cell proliferation because of their effects on 3 key cell cycle regulatory proteins, which are overexpressed in uterine leiomyomas.
Keywords: Leiomyoma; cell proliferation; fibroids; microRNA.
Conflict of interest statement
Figures

References
-
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

