Pilot evaluation of a second-generation electronic pill box for adherence to Bedaquiline and antiretroviral therapy in drug-resistant TB/HIV co-infected patients in KwaZulu-Natal, South Africa
- PMID: 29642874
- PMCID: PMC5896111
- DOI: 10.1186/s12879-018-3080-2
Pilot evaluation of a second-generation electronic pill box for adherence to Bedaquiline and antiretroviral therapy in drug-resistant TB/HIV co-infected patients in KwaZulu-Natal, South Africa
Abstract
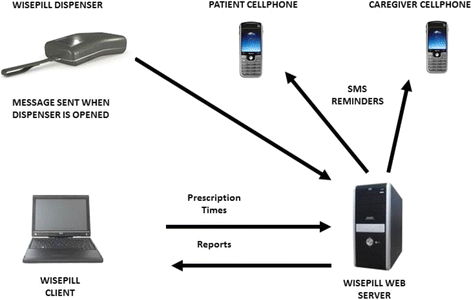
Background: The introduction of Bedaquiline, the first new antimycobacterial drug in over 40 years, has highlighted the critical importance of medication adherence in drug-resistant tuberculosis (DR-TB) treatment to prevent amplified drug-resistance and derive sustained benefit. Real-time electronic dose monitoring (EDM) accurately measures adherence and allows for titration of adherence support for anti-retroviral therapy (ART). The goal of this study was to evaluate the accuracy and acceptability of a next-generation electronic pillbox (Wisepill RT2000) for Bedaquiline-containing TB regimens.
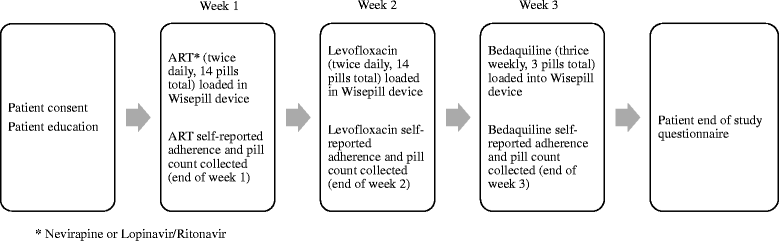
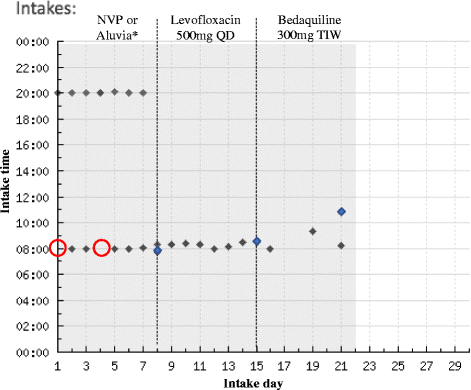
Methods: Eligible patients were DR-TB/HIV co-infected adults hospitalized for the initiation of Bedaquiline-containing treatment regimens in KwaZulu-Natal, South Africa. A one-way crossover design was used to evaluate levels of adherence and patient acceptance of EDM. Each patient was given a Wisepill device which was filled with ART, Levofloxacin or Bedaquiline over three consecutive weeks. Medication adherence was measured using Wisepill counts, patient-reported seven-day recall, and weekly pill count. An open-ended qualitative questionnaire at the end of the study evaluated participant acceptability of the Wisepill device.
Results: We enrolled 21 DR-TB/HIV co-infected inpatients admitted for the initiation of Bedaquiline from August through September 2016. In aggregate patients were similarly adherent to Bedaquiline (100%) compared to Levofloxacin (100%) and ART (98.9%) by pill count. Wisepill was more sensitive (100%) compared to seven-day recall (0%) in detecting non-adherence events (p = 0.02). Patients reported positive experiences with Wisepill and expressed willingness to use the device during a full course of DR-TB treatment. There were no concerns about stigma, confidentiality, or remote monitoring.
Conclusion: In this pilot study patients were highly adherent to Bedaquiline by all adherence measures. However, there was lower adherence to ART by pill count and Wisepill suggesting a possible challenge for adherence with ART. The use of EDM identified significantly more missed doses than seven-day recall. Wisepill was highly acceptable to DR-TB/HIV patients in South Africa, and is a promising modality to support and monitor medication adherence in complex treatment regimens.
Keywords: Bedaquiline; Drug-resistant tuberculosis; Electronic pillbox; HIV; Real-time monitoring.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards at Columbia University Medical Center and the Biomedical Research Ethics Committee at the University of KwaZulu-Natal in South Africa. Written informed consent was obtained from all patients in either isiZulu or English.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Organization WHO . Global Tuberculosis Report, 2015. Geneva: World Health Organization; 2016.
-
- (World Health Organization) WHO. Multidrug-resistant tuberculosis (MDR-TB). In: Multidrug-resistant tuberculosis (MDR-TB): 2016 update. Geneva: World Health Organization; 2016.
-
- Organization WHO . Global tuberculosis report, 2016. Geneva: World Health Organization; 2016.
-
- Gandhi NR, Andrews JR, Brust J, Montreuil R, Weissman D, Heo M, Moll A, Friedland G, Shah N. Risk factors for mortality among MDR-and XDR-TB patients in a high HIV prevalence setting. The International Journal of Tuberculosis and Lung Disease. 2012;16(1):90–97. doi: 10.5588/ijtld.11.0153. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

