Defective Base Excision Repair of Oxidative DNA Damage in Vascular Smooth Muscle Cells Promotes Atherosclerosis
- PMID: 29643057
- PMCID: PMC6053042
- DOI: 10.1161/CIRCULATIONAHA.117.033249
Defective Base Excision Repair of Oxidative DNA Damage in Vascular Smooth Muscle Cells Promotes Atherosclerosis
Abstract
Background: Atherosclerotic plaques demonstrate extensive accumulation of oxidative DNA damage, predominantly as 8-oxoguanine (8oxoG) lesions. 8oxoG is repaired by base excision repair enzymes; however, the mechanisms regulating 8oxoG accumulation in vascular smooth muscle cells (VSMCs) and its effects on their function and in atherosclerosis are unknown.
Methods: We studied levels of 8oxoG and its regulatory enzymes in human atherosclerosis, the mechanisms regulating 8oxoG repair and the base excision repair enzyme 8oxoG DNA glycosylase I (OGG1) in VSMCs in vitro, and the effects of reducing 8oxoG in VSMCs in atherosclerosis in ApoE-/- mice.
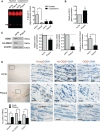
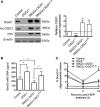
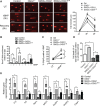
Results: Human plaque VSMCs showed defective nuclear 8oxoG repair, associated with reduced acetylation of OGG1. OGG1 was a key regulatory enzyme of 8oxoG repair in VSMCs, and its acetylation was crucial to its repair function through regulation of protein stability and expression. p300 and sirtuin 1 were identified as the OGG1 acetyltransferase and deacetylase regulators, respectively, and both proteins interacted with OGG1 and regulated OGG1 acetylation at endogenous levels. However, p300 levels were decreased in human plaque VSMCs and in response to oxidative stress, suggesting that reactive oxygen species-induced regulation of OGG1 acetylation could be caused by reactive oxygen species-induced decrease in p300 expression. We generated mice that express VSMC-restricted OGG1 or an acetylation defective version (SM22α-OGG1 and SM22α-OGG1K-R mice) and crossed them with ApoE-/- mice. We also studied ApoE-/- mice deficient in OGG1 (OGG1-/-). OGG1-/- mice showed increased 8oxoG in vivo and increased atherosclerosis, whereas mice expressing VSMC-specific OGG1 but not the acetylation mutant OGG1K-R showed markedly reduced intracellular 8oxoG and reduced atherosclerosis. VSMC OGG1 reduced telomere 8oxoG accumulation, DNA strand breaks, cell death and senescence after oxidant stress, and activation of proinflammatory pathways.
Conclusions: We identify defective 8oxoG base excision repair in human atherosclerotic plaque VSMCs, OGG1 as a major 8oxoG repair enzyme in VSMCs, and p300/sirtuin 1 as major regulators of OGG1 through acetylation/deacetylation. Reducing oxidative damage by rescuing OGG1 activity reduces plaque development, indicating the detrimental effects of 8oxoG on VSMC function.
Keywords: DNA damage; DNA glycosylases; atherosclerosis; oxidative stress; vascular diseases.
Figures





Comment in
-
Atherosclerosis linked to faulty DNA repair in VSMCs.Nat Rev Cardiol. 2018 Jul;15(7):380. doi: 10.1038/s41569-018-0021-0. Nat Rev Cardiol. 2018. PMID: 29743564 No abstract available.
References
-
- Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. - PubMed
-
- Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993;9:246–249. - PubMed
-
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. - PubMed
-
- Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 2001;88:733–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

