The plant hormone ethylene restricts Arabidopsis growth via the epidermis
- PMID: 29643073
- PMCID: PMC5924893
- DOI: 10.1073/pnas.1717649115
The plant hormone ethylene restricts Arabidopsis growth via the epidermis
Abstract
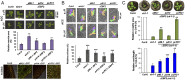
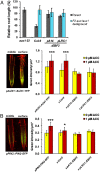
The gaseous hormone ethylene plays a key role in plant growth and development, and it is a major regulator of stress responses. It inhibits vegetative growth by restricting cell elongation, mainly through cross-talk with auxins. However, it remains unknown whether ethylene controls growth throughout all plant tissues or whether its signaling is confined to specific cell types. We employed a targeted expression approach to map the tissue site(s) of ethylene growth regulation. The ubiquitin E3 ligase complex containing Skp1, Cullin1, and the F-box protein EBF1 or EBF2 (SCFEBF1/2) target the degradation of EIN3, the master transcription factor in ethylene signaling. We coupled EBF1 and EBF2 to a number of cell type-specific promoters. Using phenotypic assays for ethylene response and mutant complementation, we revealed that the epidermis is the main site of ethylene action controlling plant growth in both roots and shoots. Suppression of ethylene signaling in the epidermis of the constitutive ethylene signaling mutant ctr1-1 was sufficient to rescue the mutant phenotype, pointing to the epidermis as a key cell type required for ethylene-mediated growth inhibition.
Keywords: Arabidopsis; EIN3 binding F-box factor EBF; auxin; ethylene; root/shoot.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189.
-
- Kieber JJ. The ethylene signal transduction pathway in Arabidopsis. J Exp Bot. 1997;48:211–218. - PubMed
-
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. - PubMed
-
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D. Ethylene in vegetative development: A tale with a riddle. New Phytol. 2012;194:895–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

