A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations
- PMID: 29643105
- PMCID: PMC5916006
- DOI: 10.1182/bloodadvances.2018015925
A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations
Abstract
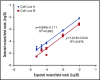
Internal tandem duplications in fms-like tyrosine kinase 3 (FLT3-ITDs) are common in acute myeloid leukemia (AML) and confer a poor prognosis. A sensitive and specific assay for the detection of minimal residual disease (MRD) in FLT3-ITD mutated AML could guide therapy decisions. Existing assays for MRD in FLT3-ITD AML have not been particularly useful because of limited sensitivity. We developed a sensitive and specific MRD assay for FLT3-ITD mutations using next-generation sequencing. The initial validation of this assay was performed by spiking fixed amounts of mutant DNA into wild-type DNA to establish a sensitivity of detection equivalent to ≥1 FLT3-ITD-containing cell in 10 000, with a minimum input of 100 000 cell equivalents of DNA. We subsequently validated the assay in bone marrow samples from patients with FLT3-ITD AML in remission. Finally, we analyzed bone marrow samples from 80 patients with FLT3-ITD relapsed/refractory AML participating in a trial of a novel FLT3 inhibitor, gilteritinib, and demonstrated a relationship between the mutation burden, as detected by the assay, and overall survival. This novel MRD assay is specific and 2 orders of magnitude more sensitive than currently available polymerase chain reaction- or next-generation sequencing-based FLT3-ITD assays. The assay is being prospectively validated in ongoing randomized clinical trials.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.J.L. has received consultancy fees and research funding from Astellas and Novartis, honoraria from Daiichi-Sankyo and Novartis, and research funding from Fujifilm and Millenium Pharmaceuticals/Takeda Pharmaceuticals. A.E.P. has received consultancy fees from Astellas, Daiichi-Sankyo, Novartis, Pfizer, and Arog Pharmaceuticals and has participated in advisory boards for Actinium Pharmaceuticals and Asana Biosciences. J.K.A has received consultancy fees from Astellas, Syros, Bristol-Myers Squibb, Celgene, Ceplene, Novartis, and Janssen Pharmaceuticals and has served as an educational speaker for the American Society of Hematology and the National Comprehensive Cancer Network. J.H., C.L., and E.B. are employees of Astellas Pharma, Inc. J.H. has ownership of Ligacept, LLC and patents (US7852995B2 [issued] and W02013163419A1 [pending]). A.R.C., J.E.M., T.T.S., V.M., and Z.X. are employees of Invivoscribe, Inc. C.D.G. declares no competing financial interests.
Figures



References
-
- Yeung DT, Mauro MJ. Prognostic significance of early molecular response in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program. 2014;2014:240-243. - PubMed
-
- Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2014;2014:244-249. - PubMed
-
- Terwijn M, van Putten WL, Kelder A, et al. . High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889-3897. - PubMed
-
- Jourdan E, Boissel N, Chevret S, et al. ; French AML Intergroup. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213-2223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

