Treatment options in idiopathic subglottic stenosis: protocol for a prospective international multicentre pragmatic trial
- PMID: 29643170
- PMCID: PMC5898326
- DOI: 10.1136/bmjopen-2018-022243
Treatment options in idiopathic subglottic stenosis: protocol for a prospective international multicentre pragmatic trial
Abstract
Introduction: Idiopathic subglottic stenosis (iSGS) is an unexplained progressive obstruction of the upper airway that occurs almost exclusively in adult, Caucasian women. The disease is characterised by mucosal inflammation and localised fibrosis resulting in life-threatening blockage of the upper airway. Because of high recurrence rates, patients with iSGS will frequently require multiple procedures following their initial diagnosis. Both the disease and its therapies profoundly affect patients' ability to breathe, communicate and swallow. A variety of treatments have been advanced to manage this condition. However, comparative data on effectiveness and side effects of the unique approaches have never been systematically evaluated. This study will create an international, multi-institutional prospective cohort of patients with iSGS. It will compare three surgical approaches to determine how well the most commonly used treatments in iSGS 'work' and what quality of life (QOL) trade-offs are associated with each approach.
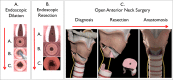
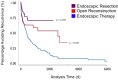
Methods and analysis: A prospective pragmatic trial comparing the 'Standard of Care' for iSGS at multiple international institutions. Patients with a diagnosis of iSGS without clinical or laboratory evidence of vasculitis or a history of endotracheal intubation 2 years prior to symptom onset will be included in the study. Prospective evaluation of disease recurrence requiring operative intervention, validated patient-reported outcome (PRO) measures as well as patient-generated health data (mobile peak flow recordings and daily steps taken) will be longitudinally tracked for 36 months. The primary endpoint is treatment effectiveness defined as time to recurrent operative procedure. Secondary endpoints relate to treatment side effects and include PRO measures in voice, swallowing, breathing and global QOL as well as patient-generated health data.
Ethics and dissemination: This protocol was approved by the local IRB Committee of the Vanderbilt University Medical Center in July 2015. The findings of the trial will be disseminated through peer-reviewed journals, national and international conference presentations and directly to patient with iSGS via social media-based support groups.
Trial registration number: NCT02481817.
Keywords: NoAAC; PR02; iSGS; pragmatic trial; subglottic stenosis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
