Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures
- PMID: 29643171
- PMCID: PMC5968459
- DOI: 10.1128/MMBR.00068-17
Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures
Abstract
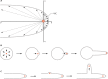
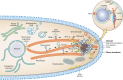
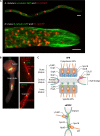
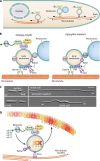
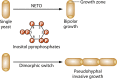
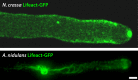
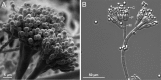
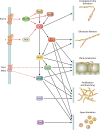
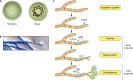
Filamentous fungi constitute a large group of eukaryotic microorganisms that grow by forming simple tube-like hyphae that are capable of differentiating into more-complex morphological structures and distinct cell types. Hyphae form filamentous networks by extending at their tips while branching in subapical regions. Rapid tip elongation requires massive membrane insertion and extension of the rigid chitin-containing cell wall. This process is sustained by a continuous flow of secretory vesicles that depends on the coordinated action of the microtubule and actin cytoskeletons and the corresponding motors and associated proteins. Vesicles transport cell wall-synthesizing enzymes and accumulate in a special structure, the Spitzenkörper, before traveling further and fusing with the tip membrane. The place of vesicle fusion and growth direction are enabled and defined by the position of the Spitzenkörper, the so-called cell end markers, and other proteins involved in the exocytic process. Also important for tip extension is membrane recycling by endocytosis via early endosomes, which function as multipurpose transport vehicles for mRNA, septins, ribosomes, and peroxisomes. Cell integrity, hyphal branching, and morphogenesis are all processes that are largely dependent on vesicle and cytoskeleton dynamics. When hyphae differentiate structures for asexual or sexual reproduction or to mediate interspecies interactions, the hyphal basic cellular machinery may be reprogrammed through the synthesis of new proteins and/or the modification of protein activity. Although some transcriptional networks involved in such reprogramming of hyphae are well studied in several model filamentous fungi, clear connections between these networks and known determinants of hyphal morphogenesis are yet to be established.
Keywords: cell wall; cytoskeleton; fungal development; hyphal morphogenesis; polarity.
Copyright © 2018 American Society for Microbiology.
Figures














References
-
- Cardwell KF, Desjardins A, Henry SH, Munkvold G, Robens J. 2001. Mycotoxins: the cost of achieving food security and food quality. APSnet Feature doi: 10.1094/APSnetFeature-2001-0901. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

