Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts
- PMID: 29643181
- PMCID: PMC5971462
- DOI: 10.1074/jbc.RA118.001739
Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts
Abstract
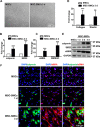
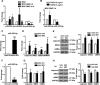
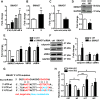
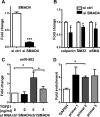
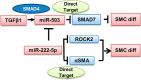
Tissue-engineered vascular grafts with long-term patency are greatly needed in the clinical settings, and smooth muscle cells (SMCs) are a critical graft component. Human mesenchymal stem cells (MSCs) are used for generating SMCs, and understanding the underlying regulatory mechanisms of the MSC-to-SMC differentiation process could improve SMC generation in the clinic. Here, we found that in response to stimulation of transforming growth factor-β1 (TGFβ1), human umbilical cord-derived MSCs abundantly express the SMC markers α-smooth muscle actin (αSMA), smooth muscle protein 22 (SM22), calponin, and smooth muscle myosin heavy chain (SMMHC) at both gene and protein levels. Functionally, MSC-derived SMCs displayed contracting capacity in vitro and supported vascular structure formation in the Matrigel plug assay in vivo More importantly, SMCs differentiated from human MSCs could migrate into decellularized mouse aorta and give rise to the smooth muscle layer of vascular grafts, indicating the potential of utilizing human MSC-derived SMCs to generate vascular grafts. Of note, microRNA (miR) array analysis and TaqMan microRNA assays identified miR-503 and miR-222-5p as potential regulators of MSC differentiation into SMCs at early time points. Mechanistically, miR-503 promoted SMC differentiation by directly targeting SMAD7, a suppressor of SMAD-related, TGFβ1-mediated signaling pathways. Moreover, miR-503 expression was SMAD4-dependent. SMAD4 was enriched at the miR-503 promoter. Furthermore, miR-222-5p inhibited SMC differentiation by targeting and down-regulating ROCK2 and αSMA. In conclusion, MSC differentiation into SMCs is regulated by miR-503 and miR-222-5p and yields functional SMCs for use in vascular grafts.
Keywords: cell differentiation; mesenchymal stem cells (MSCs); microRNA mechanism; tissue engineering; transforming growth factor beta (TGF-β); umbilical cord mesenchymal stem cells; vascular smooth muscle cells.
© 2018 Gu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Functional expression of smooth muscle-specific ion channels in TGF-β(1)-treated human adipose-derived mesenchymal stem cells.Am J Physiol Cell Physiol. 2013 Aug 15;305(4):C377-91. doi: 10.1152/ajpcell.00404.2012. Epub 2013 Jun 12. Am J Physiol Cell Physiol. 2013. PMID: 23761629 Free PMC article.
-
TGF-β1-induced differentiation of SHED into functional smooth muscle cells.Stem Cell Res Ther. 2017 Jan 23;8(1):10. doi: 10.1186/s13287-016-0459-0. Stem Cell Res Ther. 2017. PMID: 28114966 Free PMC article.
-
miR-18b inhibits TGF-β1-induced differentiation of hair follicle stem cells into smooth muscle cells by targeting SMAD2.Biochem Biophys Res Commun. 2013 Aug 30;438(3):551-6. doi: 10.1016/j.bbrc.2013.07.090. Epub 2013 Aug 2. Biochem Biophys Res Commun. 2013. PMID: 23916701
-
The role of MicroRNAs in mesenchymal stem cell differentiation into vascular smooth muscle cells.Cell Div. 2025 Mar 7;20(1):6. doi: 10.1186/s13008-025-00146-0. Cell Div. 2025. PMID: 40055797 Free PMC article. Review.
-
Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain.Mol Cell Biomech. 2006 Mar;3(1):21-34. Mol Cell Biomech. 2006. PMID: 16711069 Review.
Cited by
-
A Review on the Effect of Plant Extract on Mesenchymal Stem Cell Proliferation and Differentiation.Stem Cells Int. 2019 Jul 24;2019:7513404. doi: 10.1155/2019/7513404. eCollection 2019. Stem Cells Int. 2019. PMID: 31428160 Free PMC article. Review.
-
Glycoprotein M6B Interacts with TβRI to Activate TGF-β-Smad2/3 Signaling and Promote Smooth Muscle Cell Differentiation.Stem Cells. 2019 Feb;37(2):190-201. doi: 10.1002/stem.2938. Epub 2018 Nov 27. Stem Cells. 2019. PMID: 30372567 Free PMC article.
-
Effects of norepinephrine‑induced activation of rat vascular adventitial fibroblasts on proliferation and migration of BMSCs involved in vascular remodeling.Exp Ther Med. 2023 May 3;25(6):290. doi: 10.3892/etm.2023.11989. eCollection 2023 Jun. Exp Ther Med. 2023. PMID: 37206559 Free PMC article.
-
The application of umbilical cord-derived MSCs in cardiovascular diseases.J Cell Mol Med. 2021 Sep;25(17):8103-8114. doi: 10.1111/jcmm.16830. Epub 2021 Aug 11. J Cell Mol Med. 2021. PMID: 34378345 Free PMC article. Review.
-
Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells.Polymers (Basel). 2022 Apr 11;14(8):1555. doi: 10.3390/polym14081555. Polymers (Basel). 2022. PMID: 35458303 Free PMC article.
References
-
- Karamariti E., Margariti A., Winkler B., Wang X., Hong X., Baban D., Ragoussis J., Huang Y., Han J. D., Wong M. M., Sag C. M., Shah A. M., Hu Y., and Xu Q. (2013) Smooth muscle cells differentiated from reprogrammed embryonic lung fibroblasts through DKK3 signaling are potent for tissue engineering of vascular grafts. Circ. Res. 112, 1433–1443 10.1161/CIRCRESAHA.111.300415 - DOI - PubMed
-
- Campagnolo P., Tsai T. N., Hong X., Kirton J. P., So P. W., Margariti A., Di Bernardini E., Wong M. M., Hu Y., Stevens M. M., and Xu Q. (2015) c-Kit+ progenitors generate vascular cells for tissue-engineered grafts through modulation of the Wnt/Klf4 pathway. Biomaterials 60, 53–61 10.1016/j.biomaterials.2015.04.055 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

