Voltage and pH sensing by the voltage-gated proton channel, HV1
- PMID: 29643227
- PMCID: PMC5938591
- DOI: 10.1098/rsif.2018.0108
Voltage and pH sensing by the voltage-gated proton channel, HV1
Abstract
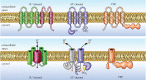
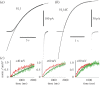
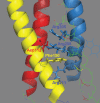
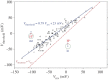
Voltage-gated proton channels are unique ion channels, membrane proteins that allow protons but no other ions to cross cell membranes. They are found in diverse species, from unicellular marine life to humans. In all cells, their function requires that they open and conduct current only under certain conditions, typically when the electrochemical gradient for protons is outwards. Consequently, these proteins behave like rectifiers, conducting protons out of cells. Their activity has electrical consequences and also changes the pH on both sides of the membrane. Here we summarize what is known about the way these proteins sense the membrane potential and the pH inside and outside the cell. Currently, it is hypothesized that membrane potential is sensed by permanently charged arginines (with very high pKa) within the protein, which results in parts of the protein moving to produce a conduction pathway. The mechanism of pH sensing appears to involve titratable side chains of particular amino acids. For this purpose their pKa needs to be within the operational pH range. We propose a 'counter-charge' model for pH sensing in which electrostatic interactions within the protein are selectively disrupted by protonation of internally or externally accessible groups.
Keywords: ion channels; pH; proton conduction; proton transport; voltage gating.
© 2018 The Author.
Conflict of interest statement
I declare I have no competing interests.
Figures
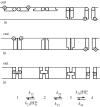
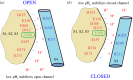
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
