The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy
- PMID: 29643230
- PMCID: PMC6364295
- DOI: 10.1126/scitranslmed.aan3464
The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy
Abstract
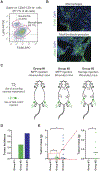
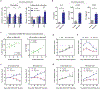
Patients undergoing surgical resection of primary breast tumors confront a risk for metastatic recurrence that peaks sharply 12 to 18 months after surgery. The cause of early metastatic relapse in breast cancer has long been debated, with many ascribing these relapses to the natural progression of the disease. Others have proposed that some aspect of surgical tumor resection triggers the outgrowth of otherwise-dormant metastases, leading to the synchronous pattern of relapse. Clinical data cannot distinguish between these hypotheses, and previous experimental approaches have not provided clear answers. Such uncertainty hinders the development and application of therapeutic approaches that could potentially reduce early metastatic relapse. We describe an experimental model system that definitively links surgery and the subsequent wound-healing response to the outgrowth of tumor cells at distant anatomical sites. Specifically, we find that the systemic inflammatory response induced after surgery promotes the emergence of tumors whose growth was otherwise restricted by a tumor-specific T cell response. Furthermore, we demonstrate that perioperative anti-inflammatory treatment markedly reduces tumor outgrowth in this model, suggesting that similar approaches might substantially reduce early metastatic recurrence in breast cancer patients.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
On the cutting edge.Nat Rev Cancer. 2018 Jul;18(7):404-405. doi: 10.1038/s41568-018-0017-4. Nat Rev Cancer. 2018. PMID: 29713084 No abstract available.
References
-
- Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, Goldhirsch A, Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J. Clin. Oncol 34, 927–935 (2016). - PMC - PubMed
-
- Cheng L, Swartz MD, Zhao H, Kapadia AS, Lai D, Rowan PJ, Buchholz TA, Giordano SH, Hazard of recurrence among women after primary breast cancer treatment—A 10-year follow-up using data from SEER-medicare. Cancer Epidemiol. Biomarkers Prev. 21, 800–809 (2012). - PubMed
-
- Pantel K, Brakenhoff RH, Brandt B, Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

