Developing and Evaluating Inhibitors against the RNase H Active Site of HIV-1 Reverse Transcriptase
- PMID: 29643235
- PMCID: PMC6002700
- DOI: 10.1128/JVI.02203-17
Developing and Evaluating Inhibitors against the RNase H Active Site of HIV-1 Reverse Transcriptase
Abstract
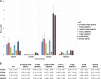
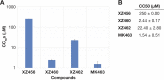
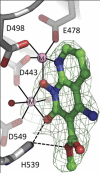
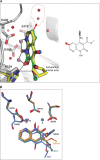
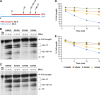
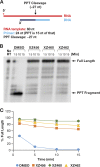
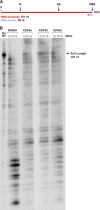
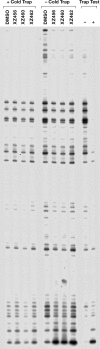
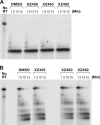
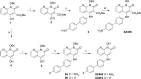
We tested three compounds for their ability to inhibit the RNase H (RH) and polymerase activities of HIV-1 reverse transcriptase (RT). A high-resolution crystal structure (2.2 Å) of one of the compounds showed that it chelates the two magnesium ions at the RH active site; this prevents the RH active site from interacting with, and cleaving, the RNA strand of an RNA-DNA heteroduplex. The compounds were tested using a variety of substrates: all three compounds inhibited the polymerase-independent RH activity of HIV-1 RT. Time-of-addition experiments showed that the compounds were more potent if they were bound to RT before the nucleic acid substrate was added. The compounds significantly inhibited the site-specific cleavage required to generate the polypurine tract (PPT) RNA primer that initiates the second strand of viral DNA synthesis. The compounds also reduced the polymerase activity of RT; this ability was a result of the compounds binding to the RH active site. These compounds appear to be relatively specific; they do not inhibit either Escherichia coli RNase HI or human RNase H2. The compounds inhibit the replication of an HIV-1-based vector in a one-round assay, and their potencies were only modestly decreased by mutations that confer resistance to integrase strand transfer inhibitors (INSTIs), nucleoside analogs, or nonnucleoside RT inhibitors (NNRTIs), suggesting that their ability to block HIV replication is related to their ability to block RH cleavage. These compounds appear to be useful leads that can be used to develop more potent and specific compounds.IMPORTANCE Despite advances in HIV-1 treatment, drug resistance is still a problem. Of the four enzymatic activities found in HIV-1 proteins (protease, RT polymerase, RT RNase H, and integrase), only RNase H has no approved therapeutics directed against it. This new target could be used to design and develop new classes of inhibitors that would suppress the replication of the drug-resistant variants that have been selected by the current therapeutics.
Keywords: HIV-1; RNase H; active site inhibitors; magnesium chelating; structure.
Copyright © 2018 American Society for Microbiology.
Figures
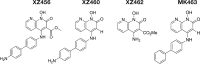




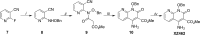
Similar articles
-
Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone.Biochemistry. 1997 Mar 18;36(11):3179-85. doi: 10.1021/bi9624696. Biochemistry. 1997. PMID: 9115994
-
Cutting into the Substrate Dominance: Pharmacophore and Structure-Based Approaches toward Inhibiting Human Immunodeficiency Virus Reverse Transcriptase-Associated Ribonuclease H.Acc Chem Res. 2020 Jan 21;53(1):218-230. doi: 10.1021/acs.accounts.9b00450. Epub 2019 Dec 27. Acc Chem Res. 2020. PMID: 31880912 Free PMC article. Review.
-
Dissecting the effects of DNA polymerase and ribonuclease H inhibitor combinations on HIV-1 reverse-transcriptase activities.Biochemistry. 2005 Feb 8;44(5):1595-606. doi: 10.1021/bi0486740. Biochemistry. 2005. PMID: 15683243
-
Inhibition of the DNA polymerase and RNase H activities of HIV-1 reverse transcriptase and HIV-1 replication by Brasenia schreberi (Junsai) and Petasites japonicus (Fuki) components.J Nat Med. 2015 Jul;69(3):432-40. doi: 10.1007/s11418-015-0885-9. Epub 2015 Feb 8. J Nat Med. 2015. PMID: 25663480
-
Novel HIV-1 reverse transcriptase inhibitors.Virus Res. 2008 Jun;134(1-2):171-85. doi: 10.1016/j.virusres.2008.01.003. Epub 2008 Mar 4. Virus Res. 2008. PMID: 18308412 Review.
Cited by
-
Pyrrolyl Pyrazoles as Non-Diketo Acid Inhibitors of the HIV-1 Ribonuclease H Function of Reverse Transcriptase.ACS Med Chem Lett. 2020 Mar 5;11(5):798-805. doi: 10.1021/acsmedchemlett.9b00617. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435387 Free PMC article.
-
Structural Insights to Human Immunodeficiency Virus (HIV-1) Targets and Their Inhibition.Adv Exp Med Biol. 2021;1322:63-95. doi: 10.1007/978-981-16-0267-2_3. Adv Exp Med Biol. 2021. PMID: 34258737
-
Exploring New Scaffolds for the Dual Inhibition of HIV-1 RT Polymerase and Ribonuclease Associated Functions.Molecules. 2021 Jun 23;26(13):3821. doi: 10.3390/molecules26133821. Molecules. 2021. PMID: 34201561 Free PMC article.
-
Targeting HIV-1 RNase H: N'-(2-Hydroxy-benzylidene)-3,4,5-Trihydroxybenzoylhydrazone as Selective Inhibitor Active against NNRTIs-Resistant Variants.Viruses. 2020 Jul 6;12(7):729. doi: 10.3390/v12070729. Viruses. 2020. PMID: 32640577 Free PMC article.
-
New targets for HIV drug discovery.Drug Discov Today. 2019 May;24(5):1139-1147. doi: 10.1016/j.drudis.2019.03.013. Epub 2019 Mar 15. Drug Discov Today. 2019. PMID: 30885676 Free PMC article. Review.
References
-
- Beilhartz GL, Wendeler M, Baichoo N, Rausch J, Le Grice S, Götte M. 2009. HIV-1 reverse transcriptase can simultaneously engage its DNA/RNA substrate at both DNA polymerase and RNase H active sites: implications for RNase H inhibition. J Mol Biol 388:462–474. doi:10.1016/j.jmb.2009.03.025. - DOI - PMC - PubMed
-
- Kirschberg TA, Balakrishnan M, Squires NH, Barnes T, Brendza KM, Chen X, Eisenberg EJ, Jin W, Kutty N, Leavitt S, Liclican A, Liu Q, Liu X, Mak J, Perry JK, Wang M, Watkins WJ, Lansdon EB. 2009. RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J Med Chem 52:5781–5784. doi:10.1021/jm900597q. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

