The Pathway To Establishing HIV Latency Is Critical to How Latency Is Maintained and Reversed
- PMID: 29643247
- PMCID: PMC6002734
- DOI: 10.1128/JVI.02225-17
The Pathway To Establishing HIV Latency Is Critical to How Latency Is Maintained and Reversed
Abstract
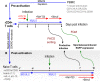
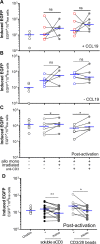
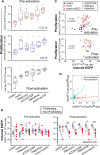
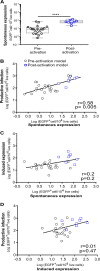
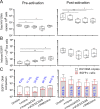
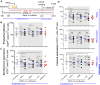
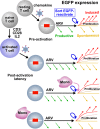
HIV infection requires lifelong antiretroviral therapy because of the persistence of latently infected CD4+ T cells. The induction of virus expression from latently infected cells occurs following T cell receptor (TCR) activation, but not all latently infected cells respond to TCR stimulation. We compared two models of latently infected cells using an enhanced green fluorescent protein (EGFP) reporter virus to infect CCL19-treated resting CD4+ (rCD4+) T cells (preactivation latency) or activated CD4+ T cells that returned to a resting state (postactivation latency). We isolated latently infected cells by sorting for EGFP-negative (EGFP-) cells after infection. These cells were cultured with antivirals and stimulated with anti-CD3/anti-CD28, mitogens, and latency-reversing agents (LRAs) and cocultured with monocytes and anti-CD3. Spontaneous EGFP expression was more frequent in postactivation than in preactivation latency. Stimulation of latently infected cells with monocytes/anti-CD3 resulted in an increase in EGFP expression compared to that for unstimulated controls using the preactivation latency model but led to a reduction in EGFP expression in the postactivation latency model. The reduced EGFP expression was not associated with reductions in the levels of viral DNA or T cell proliferation but depended on direct contact between monocytes and T cells. Monocytes added to the postactivation latency model during the establishment of latency reduced spontaneous virus expression, suggesting that monocyte-T cell interactions at an early time point postinfection can maintain HIV latency. This direct comparison of pre- and postactivation latency suggests that effective strategies needed to reverse latency will depend on how latency is established.IMPORTANCE One strategy being evaluated to eliminate latently infected cells that persist in HIV-infected individuals on antiretroviral therapy (ART) is to activate HIV expression or production with the goal of inducing virus-mediated cytolysis or immune-mediated clearance of infected cells. The gold standard for the activation of latent virus is T cell receptor stimulation with anti-CD3/anti-CD28. However, this stimulus activates only a small proportion of latently infected cells. We show clear differences in the responses of latently infected cells to activating stimuli based on how latent infection is established, an observation that may potentially explain the persistence of noninduced intact proviruses in HIV-infected individuals on ART.
Keywords: T cells; human immunodeficiency virus; latency; monocytes.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
-
- Shan L, Deng K, Gao H, Xing S, Capoferri AA, Durand CM, Rabi SA, Laird GM, Kim M, Hosmane NN. 2017. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T cells permissive for latent HIV-1 infection. Immunity 47:766–775. doi: 10.1016/j.immuni.2017.09.014. - DOI - PMC - PubMed
-
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel M-R, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy J-P, Haddad EK, Sekaly R-P. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15:893–900. doi: 10.1038/nm.1972. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

