Neutralizing Antibody Responses following Long-Term Vaccination with HIV-1 Env gp140 in Guinea Pigs
- PMID: 29643249
- PMCID: PMC6002713
- DOI: 10.1128/JVI.00369-18
Neutralizing Antibody Responses following Long-Term Vaccination with HIV-1 Env gp140 in Guinea Pigs
Abstract
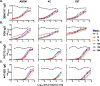
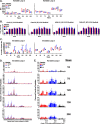
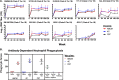
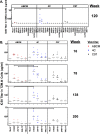
A vaccination regimen capable of eliciting potent and broadly neutralizing antibodies (bNAbs) remains an unachieved goal of the HIV-1 vaccine field. Here, we report the immunogenicity of longitudinal prime/boost vaccination regimens with a panel of HIV-1 envelope (Env) gp140 protein immunogens over a period of 200 weeks in guinea pigs. We assessed vaccine regimens that included a monovalent clade C gp140 (C97ZA012 [C97]), a tetravalent regimen consisting of four clade C gp140s (C97ZA012, 459C, 405C, and 939C [4C]), and a tetravalent regimen consisting of clade A, B, C, and mosaic gp140s (92UG037, PVO.4, C97ZA012, and Mosaic 3.1, respectively [ABCM]). We found that the 4C and ABCM prime/boost regimens were capable of eliciting greater magnitude and breadth of binding antibody responses targeting variable loop 2 (V2) over time than the monovalent C97-only regimen. The longitudinal boosting regimen conducted over more than 2 years increased the magnitude of certain tier 1 NAb responses but did not increase the magnitude or breadth of heterologous tier 2 NAb responses. These data suggest that additional immunogen design strategies are needed to induce broad, high-titer tier 2 NAb responses.IMPORTANCE The elicitation of potent, broadly neutralizing antibodies (bNAbs) remains an elusive goal for the HIV-1 vaccine field. In this study, we explored the use of a long-term vaccination regimen with different immunogens to determine if we could elicit bNAbs in guinea pigs. We found that longitudinal boosting over more than 2 years increased tier 1 NAb responses but did not increase the magnitude and breadth of tier 2 NAb responses. These data suggest that additional immunogen designs and vaccination strategies will be necessary to induce broad tier 2 NAb responses.
Keywords: HIV-1; antibody function; gp140; human immunodeficiency virus; long-term; multivalent; neutralizing antibodies; vaccine; vaccines.
Copyright © 2018 Bricault et al.
Figures


References
-
- Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi: 10.1128/JVI.01583-08. - DOI - PMC - PubMed
-
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, DeHovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83:7337–7348. doi: 10.1128/JVI.00110-09. - DOI - PMC - PubMed
-
- Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88:12623–12643. doi: 10.1128/JVI.01705-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

