MPF-based meiotic cell cycle control: Half a century of lessons from starfish oocytes
- PMID: 29643273
- PMCID: PMC5968197
- DOI: 10.2183/pjab.94.013
MPF-based meiotic cell cycle control: Half a century of lessons from starfish oocytes
Abstract
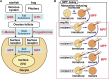
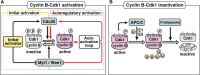
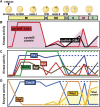
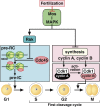
In metazoans that undergo sexual reproduction, genomic inheritance is ensured by two distinct types of cell cycle, mitosis and meiosis. Mitosis maintains the genomic ploidy in somatic cells reproducing within a generation, whereas meiosis reduces by half the ploidy in germ cells to prepare for successive generations. The meiotic cell cycle is believed to be a derived form of the mitotic cell cycle; however, the molecular mechanisms underlying both of these processes remain elusive. My laboratory has long studied the meiotic cell cycle in starfish oocytes, particularly the control of meiotic M-phase by maturation- or M phase-promoting factor (MPF) and the kinase cyclin B-associated Cdk1 (cyclin B-Cdk1). Using this system, we have unraveled the molecular principles conserved in metazoans that modify M-phase progression from the mitotic type to the meiotic type needed to produce a haploid genome. Furthermore, we have solved a long-standing enigma concerning the molecular identity of MPF, a universal inducer of M-phase both in mitosis and meiosis of eukaryotic cells.
Keywords: M-phase; MPF; cell cycle; cyclin B-Cdk1; meiosis; oocyte.
Figures




References
-
- Schleiden M.J. (1838) Beitraege zur Phytogenesis. Muller’s Arch. Anat. Physiol. Wiss. Med. 136–176.
-
- Schwann, T. (1839) Mikroskopische Untersuchungen ueber die Uebereinstimmung in der Struktur und dem Wachsthum der Thiere und Pflanzen. Verlag der Sander’schen Buchhandlung, Berlin. - PubMed
-
- Virchow, R. (1859) Die Cellular Pathologie in ihrer Begrundung auf physiologische und pathologosche Gewebelehre. Verlag von August Hirschwald, Berlin.
-
- Nurse P. (2000) A long twentieth century of the cell cycle control and beyond. Cell 100, 71–78. - PubMed
-
- Howard A., Pelc S.R. (1953) Synthesis of desoxyribonucleic acid in normal and irradiated cells and its relationship to chromosome breakage. Heredity (London) Suppl. 6, 261–273.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

