Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses
- PMID: 29643334
- PMCID: PMC5895813
- DOI: 10.1038/s41467-018-03772-1
Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses
Abstract
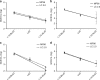
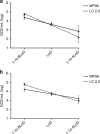
The emergence and spread of Zika virus (ZIKV) presented a challenge to the diagnosis of ZIKV infections in areas with transmission of dengue (DENV) and chikungunya (CHIKV) viruses. To facilitate detection of ZIKV infections, and differentiate these infections from DENV and CHIKV, we developed the Trioplex real-time RT-PCR assay (Trioplex assay). Here, we describe the optimization of multiplex and singleplex formats of the assay for a variety of chemistries and instruments to facilitate global standardization and implementation. We evaluated the analytical performance of all Trioplex modalities for detection of these three pathogens in serum and whole blood, and for ZIKV in urine. The limit of detection for the three viruses and in different RNA-extraction modalities is near 103 genome copy equivalents per milliliter (GCE/mL). Simultaneous testing of more than one specimen type from each patient provides a 6.4% additional diagnostic sensitivity. Overall, the high sensitivity of the Trioplex assay demonstrates the utility of this assay ascertaining Zika cases.
Conflict of interest statement
G.A.S. and J.L.M. are listed as inventors in patent application No. PCT/US2017/023021, which covers the Trioplex assay. The remaining authors declare no competing interests.
Figures



References
-
- Pan American Health Organization. Regional Zika epidemiological update (Americas) August 25, 2017. WHO http://www.paho.org/hq/index.php?option=com_content&view=article&id=1159... (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

